INTRODUCTION
Several cases of cardiac arrhythmia after coronavirus disease 2019 (COVID-19) vaccination have been reported in adults [1]. In an analysis by the Korea Disease Control and Prevention Agency (KDCA), 8,180,032 individuals aged ≤18 years has been vaccinated as of May 25, 2023. Among them, 26,722 adverse events (0.33%) including 144 suspected cases of myocarditis were reported [2]. However, no cases of cardiac arrhythmias after COVID-19 vaccination have been reported in Korean adolescents. The lipid-nanoparticle-encapsulated COVID-19 vaccine BNT162b2 (Comirnaty, Pfizer-BioNTech; Pfizer, New York, NY, USA) encodes the pre-fusion S-glycoprotein (S) of the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) [3]. This vaccine is currently under surveillance because of its unprecedented potential side effects. We report the case of a patient who developed atrial fibrillation (AF) after receiving the third dose of the BNT162b2 vaccine.
CASE REPORT
A previously healthy 18-year-old man presented at the Soonchunhyang University Bucheon Hospital with complaints of palpitations, acute chest tightness, and dyspnea. No other symptoms such as dizziness or cold sweats were noted. Two days prior to presentation, the patient received the third dose of the COVID-19 vaccine. The patient had no known allergies or history of any adverse reactions after other vaccinations.
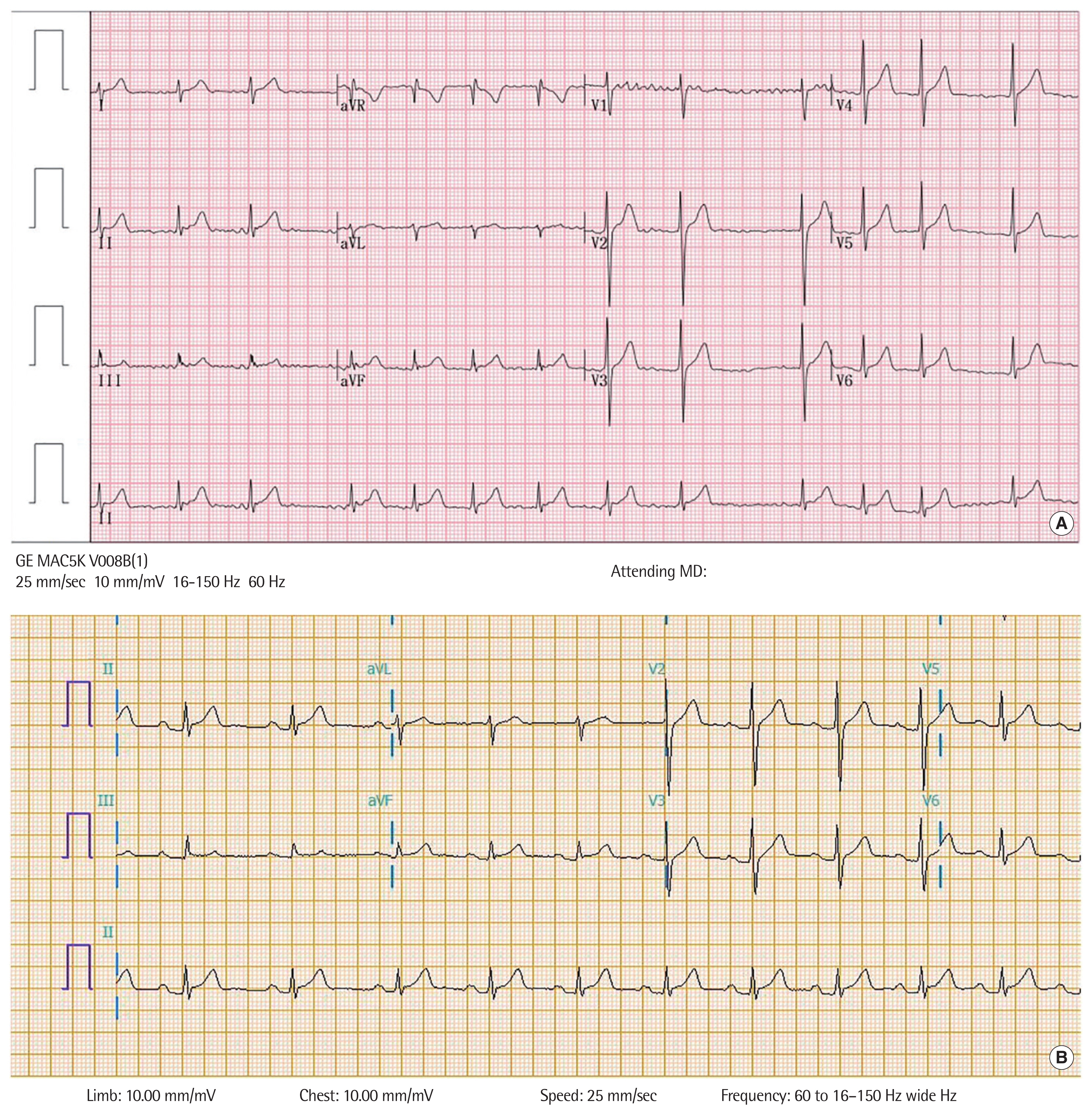
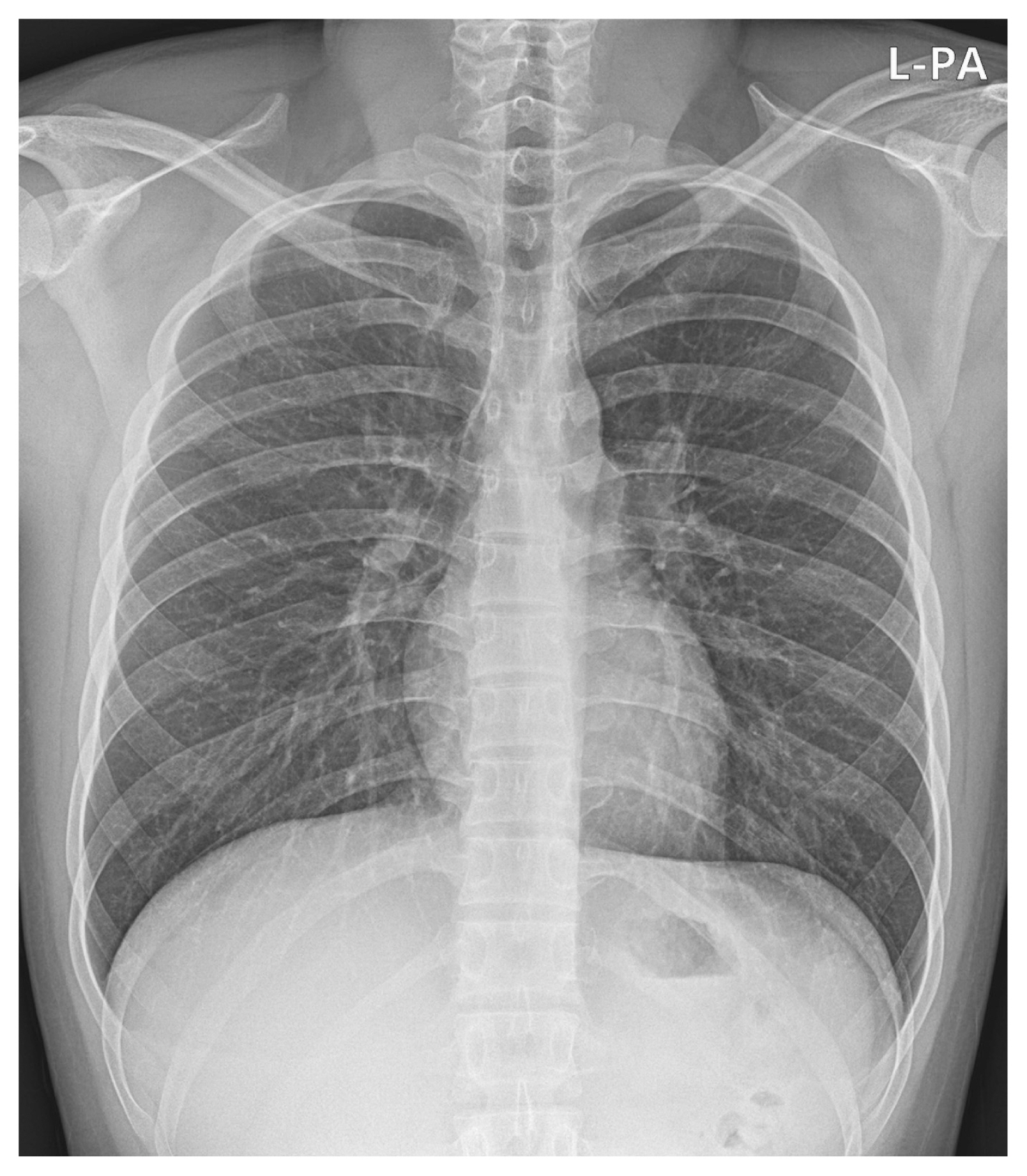
His vital signs included a heart rate of 71 beats/min, blood pressure of 113/72 mm Hg, respiratory rate of 20 breaths/min, oxygen saturation of 98% on room air, and body temperature of 36.0°C. Physical examination revealed irregular heartbeats without a cardiac murmur. A 12-lead electrocardiography (ECG) revealed AF without ST-segment elevation or depression (Fig. 1A). A chest radiograph showed a normal heart shape and size without pulmonary edema (Fig. 2). Echocardiography revealed an indeterminate left ventricular filling pattern (loss of A wave, E/e’ ratio=5.4), normal cardiac chamber size, normal cardiac function, and no pericardial effusion.
Initial laboratory tests showed an elevated N-terminal pro-B-type natriuretic peptide level of 533.0 pg/mL (reference range, <84 pg/mL). Other laboratory findings included white blood cell count, 6.26×103/μL (with 55.1% neutrophils, 32.8% lymphocytes, and 8.7% monocytes); hemoglobin level, 15.2 g/dL; C-reactive protein level, 0.15 mg/dL (reference range, <0.5 mg/dL); erythrocyte sedimentation rate, 2 mm/hr (reference range, <22 mm/hr); aspartate aminotransferase level, 19 U/L; alanine aminotransferase level, 25 U/L; creatine kinase-myocardial band level, 1.7 ng/mL (reference range, 0.1–6.3 ng/mL); troponin-T level, 0.006 ng/mL (reference range, <0.1 ng/mL); troponin-I level, 0.015 ng/mL (reference range, <0.113 ng/mL); thyroid stimulating hormone level, 2.31 μIU/mL (reference range, 0.39–5.44 μIU/mL); and free T4 level, 0.95 ng/dL (reference range, 0.8–2 ng/dL).
The patient was regularly treated at the outpatient clinic with apixaban 5 mg. Subsequently, apixaban (10 mg), flecainide (200 mg), and diltiazem (90 mg) were administered; however, no improvement was observed after 6 months.
The patient was diagnosed with persistent AF and was electrically converted to a normal sinus rhythm. Subsequently, the ECG showed a prolonged PR interval and no ST-segment deviations, and his dyspnea and palpitations disappeared (Fig. 1B). The patient was discharged without complications and remained asymptomatic without any medication for 1 year.
This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the Institutional Review Board of Soonchunhyang University Bucheon Hospital (SCHBC 2023-06-006). Written informed consent by the patient was waived due to a retrospective nature of our study.
DISCUSSION
As the number of cases of AF after COVID-19 messenger RNA vaccination is relatively small, its pathophysiology remains unknown. This illness is typically reported within 1–2 weeks of COVID-19 vaccination in patients with an average age of 50 years.
The lipid-nanoparticle-encapsulated COVID-19 vaccine BNT162b2 (Comirnaty, Pfizer-BioNTech) encodes the pre-fusion S-glycoprotein (S) of SARS-CoV-2 [3]. Commonly reported (≥10%) adverse reactions include pain at the injection site, fatigue, headache, muscle pain, chills, joint pain, fever, and swelling at the injection site. Several cases of cardiac arrhythmia after the COVID-19 vaccination have been reported in adults [1]. AF is the most commonly reported arrhythmia in patients with COVID-19; it is often new-onset, and it is associated with the worst outcomes [4]. A total of 2,611 cases of AF in vaccine recipients have been documented in the Vaccine Adverse Event Reporting System as of January 7, 2022 [5]. The mechanism by which the COVID-19 vaccine may causes adverse cardiac effects is likely complex and uncertain, and needs to be investigated further. It is possible that the immune system is dysregulated by the vaccine leading to an autoimmune reaction. Autoantibodies can damage the cardiomyocytes, causing myocarditis, ion-channel dysfunction, and arrhythmias.
In an analysis by the KDCA, 8,180,032 patients aged ≤19 years were vaccinated as of May 25, 2023. Among them, 26,722 adverse events (0.33%) and 144 suspected cases of myocarditis were reported [2]. However, cardiac arrhythmia has not been reported in children or adolescents. Therefore, vaccines are being monitored for potential side effects.
AF is rare in children and adolescents and is usually associated with structural heart disease such as dilated atria or previous intra-atrial surgery. Thyrotoxicosis, pulmonary emboli, and pericarditis should be suspected in previously healthy children who develops AF. In patients with AF for ≥48 hours, anticoagulation with warfarin is administered for 2–3 weeks to prevent systemic embolization of the atrial thrombus. Class I and III agents can be used; however, the success rate of rhythm conversion is low [6].
Persistent AF is defined as AF that persists without interruption for ≥7 days. Patients with AF for >1 year are classified as having long-standing persistent AF. Most patients who initially present with paroxysmal self-terminating AF progress to chronic forms of the arrhythmia or switch back and forth between paroxysmal and persistent or long-standing persistent AF. Management of AF after the vaccine also proceeds with the treatment process described above, such as new-onset AF. Cardioversion is required if AF persists for more than several months, as in this case.
Herein, we report the first case new-onset AF after receiving the BNT162b2 vaccine in an adolescent.