ABSTRACTTo overcome the global pandemic of coronavirus disease 2019 (COVID-19), COVID-19 vaccination has been developed and distributed. Many people have received the vaccination worldwide. However, there are some vaccinated individuals who complain of side effects due to COVID-19 vaccination. We report the case of a patient who developed adult-onset Still’s disease (AOSD) after receiving the messenger RNA COVID-19 vaccine. A 21-year-old male patient without a previous medical history developed a fever on the day of the first dose of the vaccine. He had persistent fever, arthralgia of the knee and wrist, hyperferritinemia, transient skin rash, and negative test results for rheumatoid factor or antinuclear antibody. Positron emission tomography-computed tomography scan showed lymphadenopathies with reactive patterns and no malignancy. His symptoms and laboratory abnormalities gradually improved with glucocorticoid, cyclosporine, methotrexate, and tocilizumab treatment. Although its causality is still not confirmed, AOSD should be considered in a case that meets the diagnostic criteria after COVID-19 vaccination.
INTRODUCTIONCOVID-19, also known as coronavirus disease 2019, is a highly contagious illness caused by a novel coronavirus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). COVID-19 primarily spreads through respiratory droplets when an infected person coughs, sneezes, talks, or breathes, and can also spread by touching surfaces contaminated with the virus and then touching the face. The symptoms of COVID-19 can vary from mild to severe and may appear 2 to 14 days after exposure. Common symptoms include fever, cough, fatigue, body aches, sore throat, shortness of breath, loss of taste or smell, headache, and gastrointestinal issues. However, it’s important to note that some infected individuals may remain asymptomatic or have only mild symptoms, while others may develop severe respiratory distress and complications. COVID-19 can affect individuals of all ages, but older adults and those with underlying health conditions such as cardiovascular disease, diabetes, respiratory conditions, or compromised immune systems are at a higher risk of developing severe illness or complications. To prevent the spread of COVID-19, various public health measures have been implemented, including social distancing, wearing masks, practicing good hand hygiene, and avoiding large gatherings. Additionally, testing, contact tracing, and quarantine measures have played a crucial role in controlling the transmission of the disease [1].
In addition, to overcome COVID-19 pandemic, a widespread COVID-19 vaccination has been conducted. As of March 25, 2022, approximately 10.8 billion doses of COVID-19 vaccine have been administered worldwide [2]. COVID-19 vaccines have not had enough time to undergo rigorous testing and evaluation to assess their safety and effectiveness. Clinical trials have shown that authorized vaccines provide significant protection against COVID-19, reducing the risk of severe illness, hospitalization, and death. The effectiveness of different vaccines may vary, but overall they have demonstrated high efficacy in preventing COVID-19 infection. Vaccination not only protects individuals but also contributes to community immunity (herd immunity), reducing the overall spread of the virus and protecting vulnerable populations [3].
COVID-19 vaccines have been shown to be generally safe and well-tolerated. Common side effects of COVID-19 vaccines are generally mild and temporary, including pain or swelling at the injection site, fatigue, headache, muscle pain, chills, fever, and nausea. These side effects are a sign that the immune system is responding to the vaccine and are similar to what can occur with other vaccines.Serious side effects from COVID-19 vaccines are rare. However, like any medical intervention, vaccines can have rare risks. Regulatory authorities closely monitor vaccine safety and investigate any reports of adverse events [4].
As the number of COVID-19 vaccinations increases, cases of severe side effects to messenger RNA (mRNA) vaccines, which were not reported at the time of clinical trials, are being observed [5,6]. With so many people getting vaccinated against COVID-19, the number of cases of COVID-19 side effects is increasing. Additionally, cases of individuals who have developed autoimmune diseases, presumed to be caused by hyperinflammation evoked by vaccination, have been reported. Here, we present a patient who developed adult-onset Still’s disease (AOSD) after mRNA vaccination.
CASE REPORTA 21-year-old healthy male patient developed fever several hours after receiving the first dose of the BNT162b2 mRNA COVID-19 vaccine (Pfizer Inc., New York, NY, USA) on June 24, 2021. The fever persisted for 1 week; thereafter, he visited the outpatient clinic and was admitted on July 2, 2021. His main symptoms were fever, cold sweat, sore throat, and occasional chills. After the vaccination, he experienced watery diarrhea once a day and sometimes once every 2 days; however, these symptoms improved at the time of hospitalization. After hospitalization, he complained of arthralgia in the right wrist and left knee and a rash on the forehead and both axillae.
The patient underwent a COVID-19 polymerase chain reaction test as a pre-hospital test, and it was confirmed as negative. When the antibody to COVID-19 was checked by a STANDARD Q COVID-19 immunoglobulin M (IgM)/immunoglobulin G (IgG) Plus test (SD Biosensor, Seoul, Korea) at the time the patient had symptoms, IgM was negative, and IgG was positive.
At admission, his clinical characteristics were as follows: blood pressure, 100/60 mm Hg; heart rate, 60 beats/min; respiratory rate, 16 cycles/min; and body temperature, 38.5°C. On laboratory examination, we observed the following: white blood cell count, 18,000/μL (neutrophil, 89.5%); hemoglobin, 16.3 g/dL; platelet count, 263,000/μL; creatinine level, 0.80 mg/dL (estimated glomerular filtration rate, 127.70); aspartate aminotransferase/alanine aminotransferase, 80/67 IU/L; lactate dehydrogenase, 720 U/L (reference, 0–250 U/L); ferritin, 25,635 ng/mL (reference, 30–400 ng/mL); lactic acid, 15.5 mg/dL (reference, 4.5–19.8 mg/dL); and C-reactive protein, 141.99 mg/L. Increased d-dimer (2.56 μg/mL; reference, 0–0.48 μg/mL) and fibrin degradation products (3.44 μg/mL; reference, 0–2.01 μg/mL) and prolonged activated partial thromboplastin (39.5 seconds) and prothrombin times (14.3 seconds) were observed. The rheumatoid factor (RF) and anti-nuclear antibody (ANA) tests were negative. The adenosine deaminase level was 119.0 IU/L (reference, 6.0–20.0 IU/L).
No organisms were isolated in blood, urine, and stool cultures. Infectious etiologies such as human immunodeficiency virus, hepatitis A, hepatitis B, hepatitis C, Coxiella burnetii, and Ebstein-Barr virus were excluded by serologic tests. The Mycobacterium tuberculosis release interferon-gamma test was also negative.
Chest radiography did not indicate active lung lesions and cardiomegaly. A neck computed tomography scan performed because of the sore throat symptoms showed multiple bilateral lymphadenopathies without abscess. A positron emission tomography-computed tomography scan revealed a low-level of fluorodeoxyglucose (FDG) uptake in multiple lymph node enlargements (bilateral neck level I–IV, both axillary, abdominal left para-aortic, and both external iliac areas) and a diffuse FDG uptake in the bone marrow (spine, both clavicles, scapulae, pelvic bones, and femora) and the spleen but not in the liver, which is suggestive of a reactive pattern rather than malignancy (Fig. 1).
Considering the clinical course and laboratory or imaging examinations, the patient was less likely to have a malignancy or an infection. No cardiac symptoms or abnormalities were noted; hence, the patient was diagnosed with AOSD based on the Yamaguchi criteria. Especially, he met three of the four major criteria (i.e., fever, arthralgia, and leukocytosis) and all the five minor criteria (i.e., sore throat, lymphadenopathy, splenomegaly, abnormal liver function test, and negative RF and ANA) [7]. The patient had persistent fever for >2 weeks, with associated arthralgia, splenomegaly, hyperferritinemia, neutrophil-dominant leukocytosis, abnormal liver function test, and negative results for RF and ANA. Treatment with methylprednisolone 40 mg/day promptly improved his symptoms, including fever and inflammatory markers. However, when the methylprednisolone dose was reduced to 20 mg/day, the fever recurred, and the pain in the right wrist and left knee worsened; therefore, we added cyclosporine and methotrexate administration. After that, the symptoms were aggravated once more, and tocilizumab was added. Gradually, the clinical symptoms, leukocytosis, and serum ferritin levels improved (Fig. 2).
This study was approved by the Institutional Review Board of the Soonchunhyang University Cheonan Hospital, Cheonan and the requirement for informed consent was waived by collecting anonymized data (IRB no., SCHCA 2021-11-016). The patient provided informed consent for this case report. The patient gave informed consent verbally, which will be provided in document if necessary.
DISCUSSIONAdverse events following immunization (AEFI) are any untoward, unfavorable, or unintended medical occurrence following vaccination [8]. Indeed, after the COVID-19 vaccination, various AEFIs have been reported, ranging from local side effects, such as injection site redness and pain to fever, generalized myalgia, and multisystem inflammatory syndrome (MIS) [9,10]. In particular, cytokine release is induced by the activation of T cell immunity by the COVID-19 vaccine [11]; this immune response can cause autoimmune disease caused by the COVID-19 vaccine. Vaccines can exert a protective effect by inducing an adaptive immune response that can stimulate a state of hyperinflammation. Following vaccination, healthy individuals show dramatic increases in type I interferon expression, oxidative stress and accumulation of DNA damage in blood cells, as well as the production of potent anti-SARS-CoV-2 neutralising antibodies [12]. ANCA-associated vasculitis and autoimmune hepatitis after mRNA vaccination have been reported [13,14].
Vaccine-associated autoimmunity is a widely known phenomenon. Autoimmune diseases attributed to vaccination include Gillian-Barre syndrome after swine influenza vaccination; idiopathic thrombocytopenic purpura after measles, mumps, and rubella vaccination; myopericarditis after smallpox vaccination; and multiple sclerosis suspected to be associated with hepatitis B vaccination [15]. Interestingly, mRNA may stimulate innate immunity by activating pattern recognition receptors, and lipid nanoparticles are highly inflammatory, which confer their potent adjuvant activity to induce an adaptive immune response [6,7]. New-onset or flare-up of immune-mediated disease (IMD) has been reported after COVID-19 vaccination: 27 IMD cases, of which 10 new-onset were following BNT162b2 mRNA vaccination [16]. Also, a fewr cases of MIS have been reported after COVID-19 vaccination [17].
AOSD is a representative disease of non-familial systemic autoinflammatory disorder characterized by a cytokine storm that probably starts with activation of the innate immune system [18]. We managed a case of a young man who had no specific medical history who developed systemic inflammation after receiving BNT162b2 mRNA COVID-19 vaccination. There was a high serum ferritin level and no evidence of cardiac involvement and hypotension; therefore, he was diagnosed with AOSD rather than MIS-adult, although he experienced minor gastrointestinal symptoms.
Previously, the onset of AOSD following influenza vaccination was reported in two cases, where symptoms developed within 2 days after vaccination [19,20]. There were nine case reports of patients who developed new onset AOSD after receiving the COVID-19 vaccine [21–29]. We have summarized these cases in Table 1. The mean age of the patients was 37.8 years (minimum, 20 years; maximum, 56 years), three were male and six were female. The patients developed symptoms on average 19 days after vaccination (minimum, 4 days; maximum, 90 days), and subsequently, six patients developed the first symptoms after the first dose and three patients after the second dose. Symptoms developed after inoculation with ChAdOx1 nCoV-19 in three cases, BNT162b2 in five cases, and mRNA-1273 in one case. Most of them were treated with steroid pulse therapy, and three cases were treated with tocilizumab. All patients improved their clinical course after treatment, and there were no deaths.
We experienced a case of AOSD after COVID-19 mRNA vaccination. AOSD is an autoinflammatory disease where intense activation of innate immune cells and overproduction of proinflammatory cytokines are key pathogeneses. Although further studies are needed to confirm whether these pathogeneses can be triggered by COVID-19 mRNA vaccination, AOSD should be considered in cases that meet the diagnostic criteria after COVID-19 vaccination.
ACKNOWLEDGMENTSThe authors thank all medical staff in Intensive care units for treating all COVID-19 patients and patients who suffer from ADFI due to COVID-19 vaccinations. This work was supported by Soonchunhyang University/Soonchunhyang University Cheonan Hospital. The funder provides funding for English translations and paper submissions.
Fig. 1(A) Patient’s positron emission tomography-computed tomography (PET-CT) findings. (B, C) A PET-CT scan showed low-level fluorodeoxyglucose uptake in multiple lymph node enlargements and diffuse uptake in the bone marrow and the spleen. 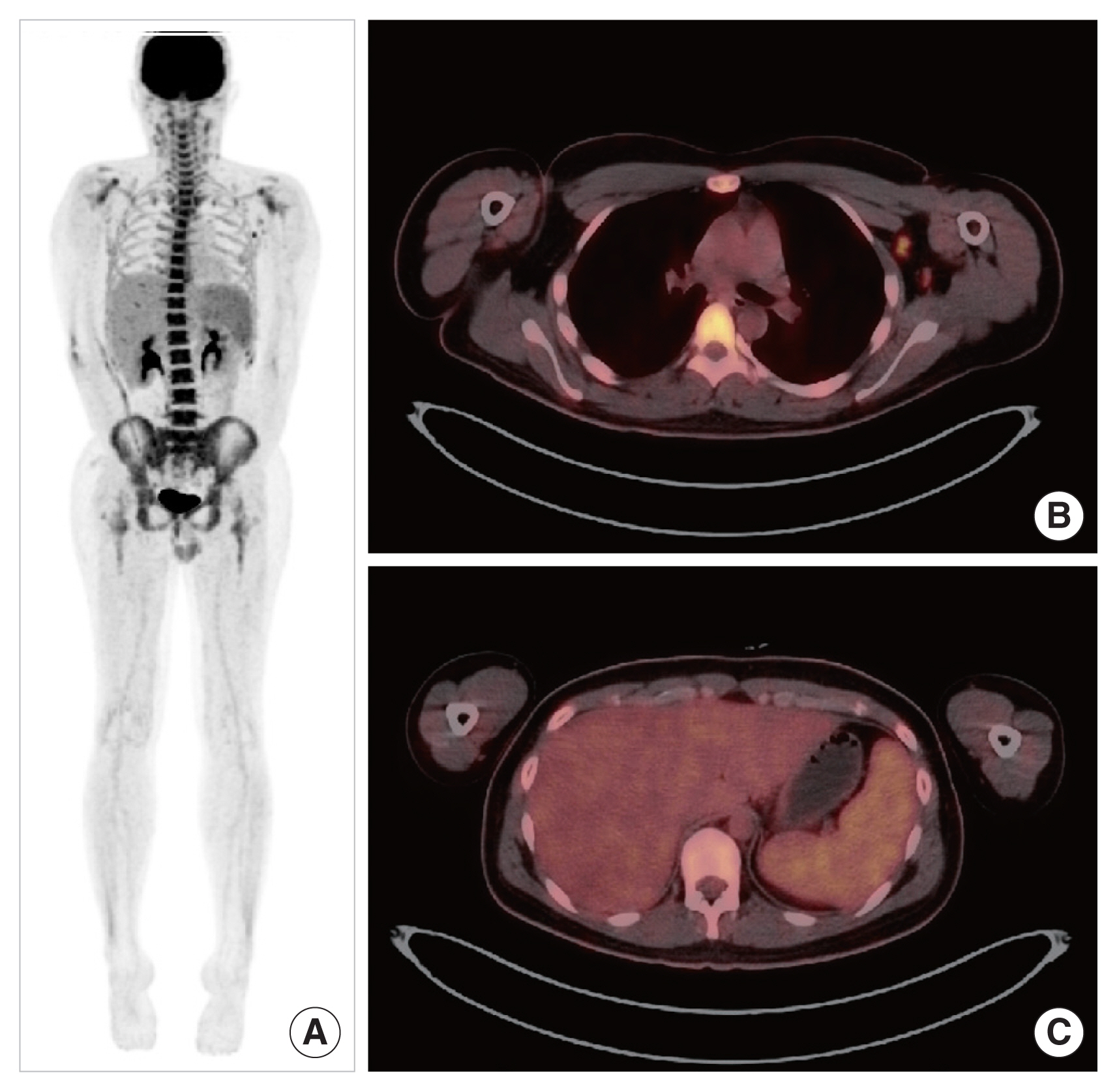
Fig. 2(A, B) Patient’s clinical course and use of therapeutics. Fever, leukocytosis, and hyperferritinemia were improved after starting steroid treatment. However, when the steroid dose was reduced, the clinical findings worsened again; therefore, cyclosporine and methotrexate were administered. HAD, hospital admission days; WBC, white blood cell; LDH, lactate dehydrogenase; CRP, C-reactive protein. 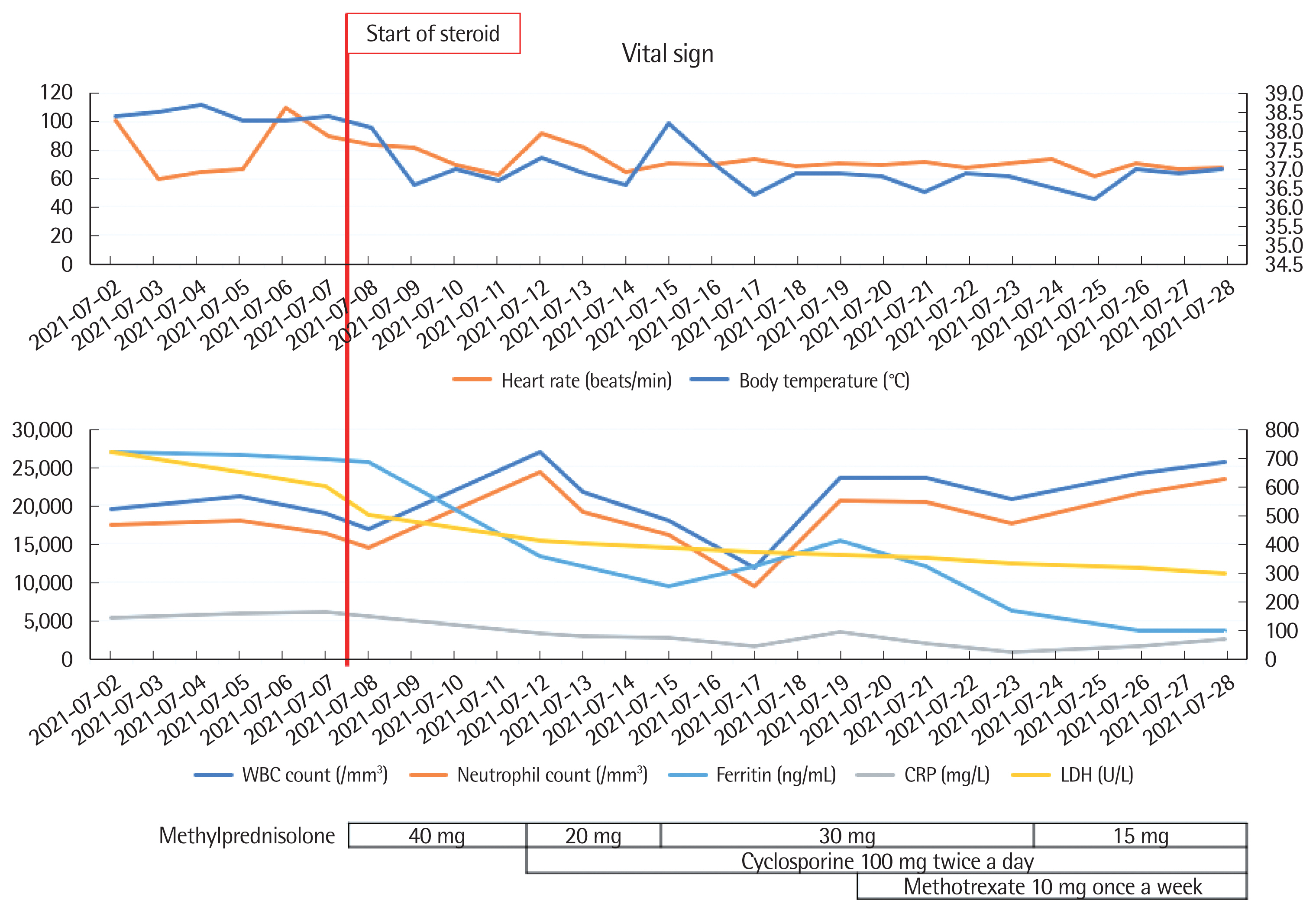
Table 1Review of literatures of adult-onset Still’s disease cases after COVID-19 vaccination COVID-19, coronavirus disease 2019; M, male; F, female; LFT, liver function test; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; ECG, electrocardiogram; IL-1, interleukin-1; IV, intravenous; LDH, lactate dehydrogenase; CT, computed tomography; PET-CT, positron emission tomography/computed tomography. REFERENCES2. COVID-19 dashboard by the Center for Systems Science and Engineering at Johns Hopkins University [Internet]. Baltimore (MD): Johns Hopkins University & Medicine; 2021. [cited 2022 Mar 25]. Available from: https://coronavirus.jhu.edu/map.html
3. Grana C, Ghosn L, Evrenoglou T, Jarde A, Minozzi S, Bergman H, et al. Efficacy and safety of COVID-19 vaccines. Cochrane Database Syst Rev 2022;12: CD015477.
4. Fiolet T, Kherabi Y, MacDonald CJ, Ghosn J, Peiffer-Smadja N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin Microbiol Infect 2022;28: 202-21.
5. Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med 2020;383: 2603-15.
6. Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384: 403-16.
7. Yamaguchi M, Ohta A, Tsunematsu T, Kasukawa R, Mizushima Y, Kashiwagi H, et al. Preliminary criteria for classification of adult Still’s disease. J Rheumatol 1992;19: 424-30.
8. Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol 2021;21: 195-7.
9. Zhang C, Maruggi G, Shan H, Li J. Advances in mRNA vaccines for infectious diseases. Front Immunol 2019;10: 594.
10. World Health Organization. Global manual on surveillance of adverse events following immunization [Internet]. Geneva: World Health Organization; 2014. [cited 2022 Mar 25]. Available from: https://apps.who.int/iris/handle/10665/206144
11. MacDonald N, McNeil SA, Comeau J, Harmon S, Dube E, Bucci LM. Surveillance for COVID-19 vaccine effectiveness and serious adverse events following immunization. Ottawa (ON): CANVax; 2020.
12. Kaur RJ, Dutta S, Bhardwaj P, Charan J, Dhingra S, Mitra P, et al. Adverse events reported from COVID-19 vaccine trials: a systematic review. Indian J Clin Biochem 2021;36: 427-39.
13. Au L, Fendler A, Shepherd ST, Rzeniewicz K, Cerrone M, Byrne F, et al. Cytokine release syndrome in a patient with colorectal cancer after vaccination with BNT162b2. Nat Med 2021;27: 1362-6.
14. Ntouros PA, Vlachogiannis NI, Pappa M, Nezos A, Mavragani CP, Tektonidou MG, et al. Effective DNA damage response after acute but not chronic immune challenge: SARS-CoV-2 vaccine versus Systemic Lupus Erythematosus. Clin Immunol 2021;229: 108765.
15. Shakoor MT, Birkenbach MP, Lynch M. ANCA-associated vasculitis following Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis 2021;78: 611-3.
16. Bril F, Al Diffalha S, Dean M, Fettig DM. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: causality or casualty? J Hepatol 2021;75: 222-4.
17. Salemi S, D’Amelio R. Could autoimmunity be induced by vaccination? Int Rev Immunol 2010;29: 247-69.
18. Watad A, De Marco G, Mahajna H, Druyan A, Eltity M, Hijazi N, et al. Immune-mediated disease flares or new-onset disease in 27 subjects following mRNA/DNA SARS-CoV-2 vaccination. Vaccines (Basel) 2021;9: 435.
19. Park JW, Yu SN, Chang SH, Ahn YH, Jeon MH. Multisystem inflammatory syndrome in an adult after COVID-19 vaccination: a case report and literature review. J Korean Med Sci 2021;36: e312.
20. Vogel TP, Top KA, Karatzios C, Hilmers DC, Tapia LI, Moceri P, et al. Multisystem inflammatory syndrome in children and adults (MIS-C/A): case definition & guidelines for data collection, analysis, and presentation of immunization safety data. Vaccine 2021;39: 3037-49.
21. Yoshioka K, Fujimoto S, Oba H, Minami M, Aoki T. Onset of adult-onset Still’s disease following influenza vaccination. Mod Rheumatol 2011;21: 432-5.
22. Yoo WH. Adult onset Still’s disease following influenza vaccination. Joint Bone Spine 2010;77: 373-4.
23. Leone F, Cerasuolo PG, Bosello SL, Verardi L, Fiori E, Cocciolillo F, et al. Adult-onset Still’s disease following COVID-19 vaccination. Lancet Rheumatol 2021;3: e678-80.
24. Magliulo D, Narayan S, Ue F, Boulougoura A, Badlissi F. Adult-onset Still’s disease after mRNA COVID-19 vaccine. Lancet Rheumatol 2021;3: e680-2.
25. Baicus C, Delcea C, Pinte L, Dan GA. Hyper-inflammation after COVID-19 mARN vaccination: at the crossroads of multisystem inflammatory disease and adult-onset Still’s disease. Does terminology matter? Rom J Intern Med 2022;60: 3-5.
26. Sharabi A, Shiber S, Molad Y. Adult-onset Still’s disease following mRNA COVID-19 vaccination. Clin Immunol 2021;233: 108878.
27. Park SY, Lee KH. Adult-onset Still’s disease after BNT162b2 mRNA COVID-19 vaccine. J Korean Med Sci 2021;36: e344.
|
|
|||||||||||||||||||||||||||||||||||||