INTRODUCTION
Dermatomyositis (DM) affects skeletal muscles, including those involved in swallowing, leading to the presence of dysphagia [1]. Remission of dysphagia typically coincides with remission of DM [2–4]. Coronavirus disease 2019 (COVID-19) patients demonstrated a higher incidence of dysphagia compared to non-COVID-19 patients [5]. Currently, no studies or case reports have been reported on the coexistence of COVID-19 and its impact on dysphagia in DM patients. This is the first case report addressing the impact of COVID-19 on dysphagia in a patient with DM.
CASE REPORT
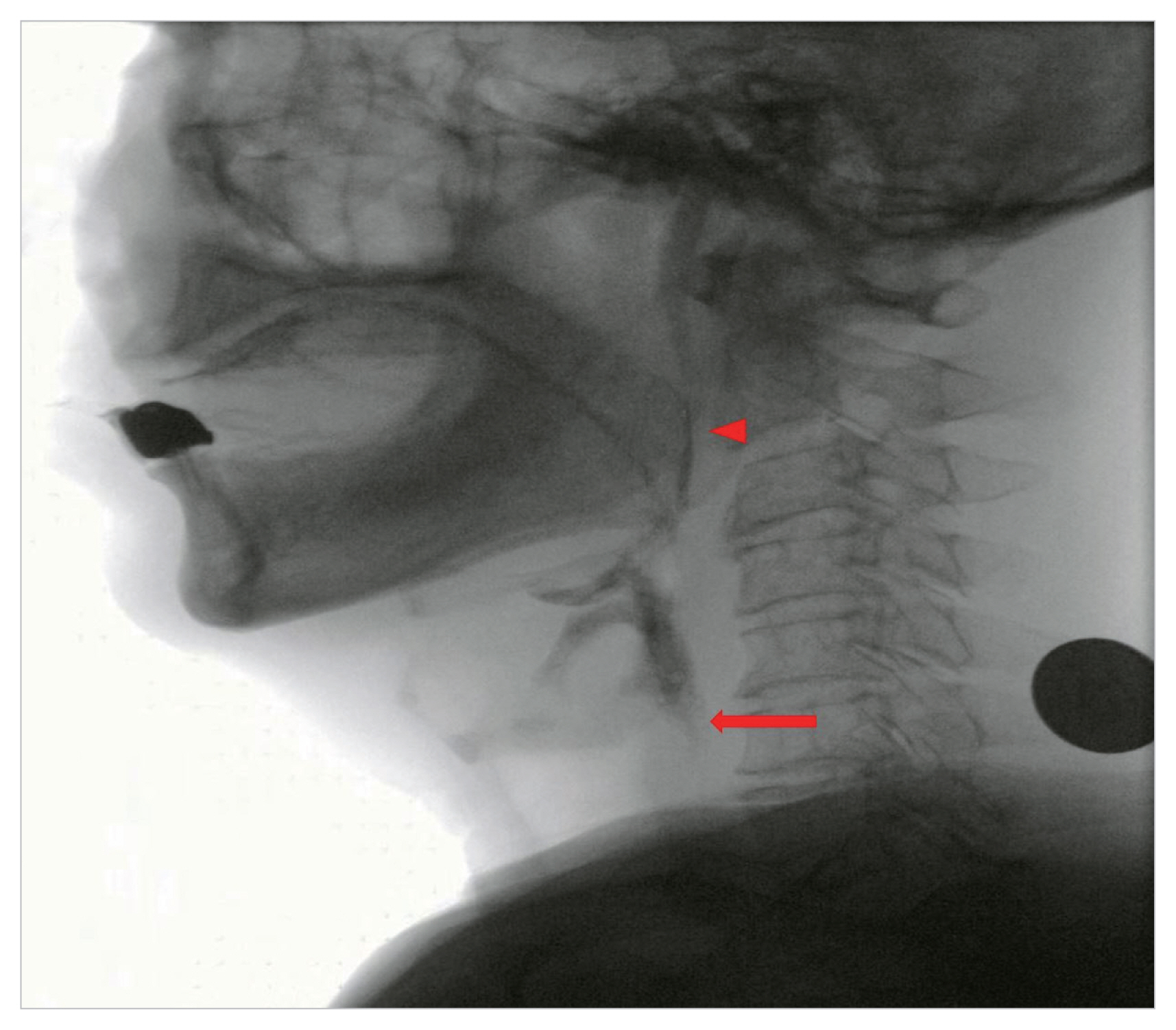
A 65-year-old male with a medical history of renal cell carcinoma (status post partial nephrectomy) and non-small cell lung cancer (status post concomitant chemoradiotherapy) presented with complaints of dysphagia. The patient reported experiencing a sensation of food sticking in the pharynx for about 2 months and proximal muscle weakness in both upper and lower extremities for about 1 month. He had difficulty swallowing both liquids and solid foods. Physical examination revealed a skin rash on the face and neck. The patient exhibited symmetric proximal muscle weakness, with a power of 4/5 in the upper extremities and 3/5 in the lower extremities. Laboratory findings showed an elevated serum creatine kinase (CK) level of 363 U/L (reference value, 20–171 U/L). A videofluoroscopic swallowing study (VFSS) conducted on the third day of admission revealed food accumulation in the lateral sulcus, decreased food propulsion posteriorly during the oral phase. Additionally, there was a delayed swallowing reflex, incomplete closure of the velopharyngeal port, decreased pharyngeal peristalsis, decreased elevation of the larynx, impaired closure of the larynx, reduced upper esophageal sphincter (UES) opening, delayed pharyngeal transit time, and a large amount of vallecular and piriform sinus residue during the pharyngeal phase (Fig. 1). Therefore, a nasogastric tube was inserted to provide enteral feeding.
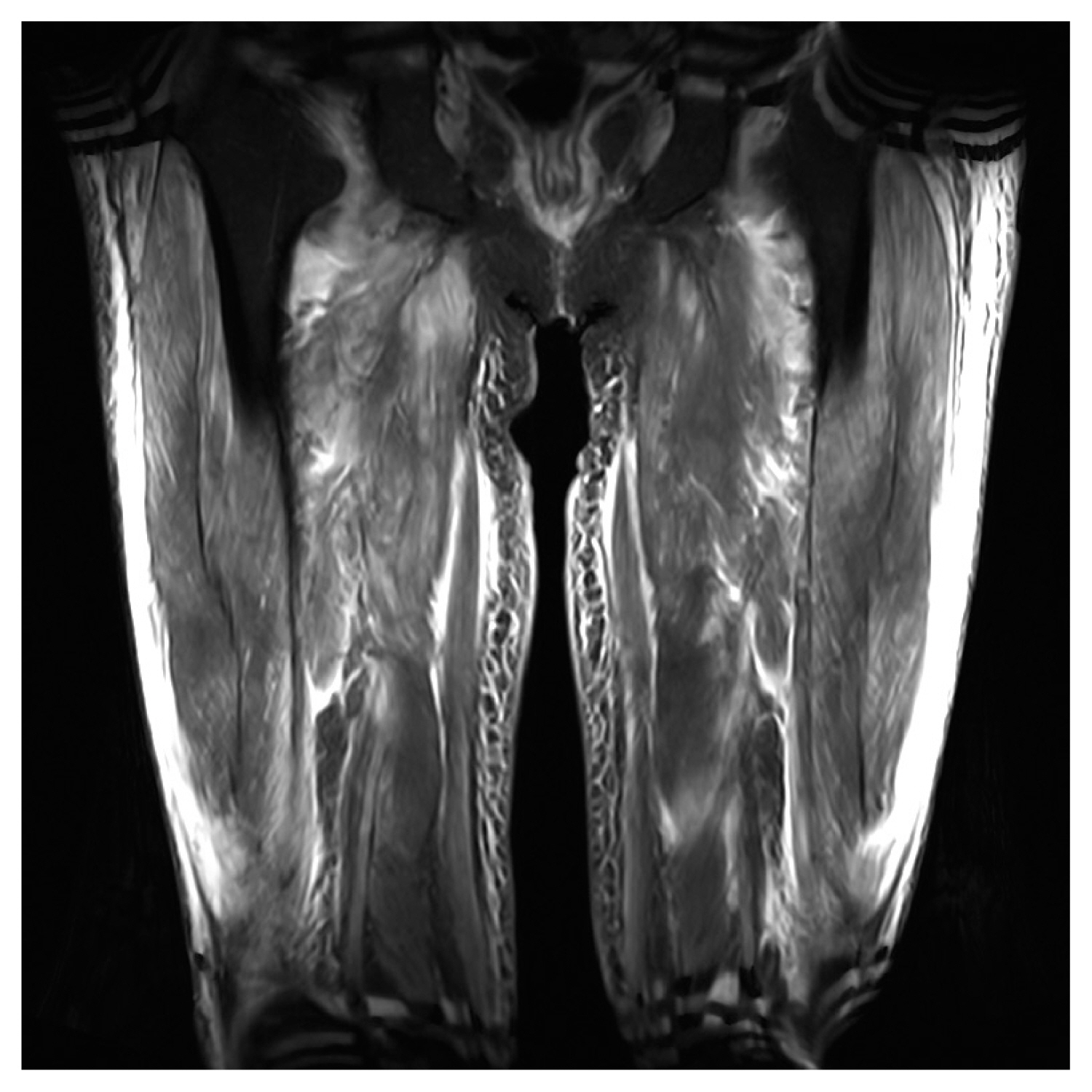
A thigh magnetic resonance imaging revealed multiple areas of increased T2 signal intensity in the proximal thigh muscles, including the vastus, rectus femoris, gracilis, sartorius, and semimembranosus muscles (Figs. 2, 3). Electromyography/nerve conduction studies and a muscle biopsy of the right vastus medialis were performed, leading to a diagnosis of DM. The patient was treated with intravenous methylprednisolone and oral prednisolone, resulting in a positive response. After 1.5 months of hospitalization, the weakened proximal muscle power improved to a grade of 4/5, and the CK level normalized.
However, one month after admission, the patient contracted COVID-19. Chest computed tomography (CT) conducted one day prior to COVID-19 confirmation revealed findings of pneumonia and pleural effusion in both lungs. On the 24th day of COVID-19, a follow-up chest CT showed decreased pleural effusion. No findings of interstitial lung disease were observed on the chest CT. The patient did not require intubation, tracheostomy, or ventilator care. Due to the improvement in muscle power and CK level observed after 1.5 months of hospitalization and on the 15th day of COVID-19 diagnosis, a follow-up VFSS was conducted on the 19th day of COVID-19 to assess the progress of dysphagia recovery. When compared to the VFSS conducted prior to the COVID-19 diagnosis, improvement was observed in the oral phase, with no food accumulation in the lateral sulcus and improved posterior food propulsion. In the pharyngeal phase, there was better UES opening and increased larynx elevation. However, the velum and uvula did not make contact with the posterior pharyngeal wall, nor did the base of the tongue and pharyngeal wall make contact (Fig. 4). A large amount of residue in the vallecular and piriform sinuses, as well as food aspiration, were observed. Despite the improvement in muscle power and normalization of CK levels following steroid treatment, the VFSS results necessitated the continuation of nasogastric tube feeding.
Informed consent was omitted as the patient’s personal information, including identifiable images, was not disclosed.
DISCUSSION
DM is a rare autoimmune disease that primarily affects the muscles and skin. It is characterized by muscle weakness, skin rash, and inflammation. The most common symptoms of DM include muscle weakness, particularly in the proximal muscles such as the shoulders, hips, and thighs [6]. In addition to muscle weakness, patients may experience skin changes, including a characteristic rash that appears on the face, neck, chest, back, and hands.
Patients with DM may have an increased risk of developing complications if they contract COVID-19 compared to the general population. This is because DM is an autoimmune disease that can weaken the immune system [7]. A compromised immune system can make it more difficult for the body to fight off infections, including viral infections like COVID-19. Additionally, some individuals with DM may be taking immunosuppressive medications to manage their condition, which can further increase the risk of infection and complications from COVID-19.
Dysphagia can occur in approximately 30%–40% of patients with DM [2]. In DM, dysphagia is often associated with weakness in the muscles involved in swallowing [8], such as the throat muscles and upper third of the esophagus. It can result in difficulty swallowing both liquids and solid foods and may contribute to weight loss and malnutrition in some cases. Prompt recognition and management of dysphagia in DM are important to ensure adequate nutrition and prevent complications related to swallowing difficulties.
In the VFSS results of this case report, conducted prior to the patient with DM being diagnosed with COVID-19, findings showed food accumulation in the lateral sulcus and decreased food propulsion posteriorly during the oral phase. This was attributed to decreased tension in the lips and cheeks, as well as diminished tongue movement. In a normal swallow, the larynx moves anteriorly and superiorly by the suprahyoid muscles, which leads to the downward rotation of the epiglottis, resulting in the closure of the laryngeal inlet. This action also pulls the anterior wall of the pharynx, causing relaxation of the cricopharyngeal muscle and opening of the UES, increasing the diameter of the pharynx and facilitating the passage of food. However, in this case, findings indicated a decreased elevation of the larynx, which is believed to be due to weakness in the suprahyoid muscles. As a result, impaired closure of the larynx, reduced UES opening, and a significant amount of residue in the vallecular and piriform sinuses were observed.
The oropharynx and upper third of the esophagus are composed of skeletal muscle tissue, which can be inflamed in DM and lead to dysphagia. The remission of dysphagia usually occurs simultaneously with the remission of DM [2–4]. The absence of evidence of myositis disease activity was defined as having normal muscle strength, and normal levels of muscle enzymes, including CK [9].
The characterization of dysphagia and laryngeal findings in COVID-19 patients includes pooling of secretions, silent aspiration, residue after swallowing in the vallecula and hypopharynx, impaired vocal cord movement, erythema of the vocal folds, and edema in the arytenoid region [10]. The proposed mechanisms of dysphagia after COVID-19 include disruptions in the peripheral and central nervous systems, intubation, debility, and pulmonary dysfunction [11].
In this case report, a VFSS conducted after COVID-19 showed no food accumulation in the lateral sulcus and improved posterior food propulsion. It was observed that larynx elevation was better and UES opening was also improved, which was believed to be the result of the improvement in DM, leading to the strengthening of the tongue, facial muscles, and suprahyoid muscles. During a normal swallow, the velum and uvula prevent food from refluxing into the nasal cavity. Additionally, the base of the tongue and pharyngeal wall make contact, generating pressure to ensure the passage of food into the pharynx without residue. However, in this case, findings revealed that the velum and uvula did not make contact with the posterior pharyngeal wall, nor did the base of the tongue and pharyngeal wall make contact, which was not observed prior to the diagnosis of COVID-19. This resulted in a large amount of residue in the vallecular and piriform sinuses, leading to food aspiration.
Before COVID-19 infection, the main problem was difficulty in UES opening due to weakness in the suprahyoid muscles. After the COVID-19 infection, the UES opening improved, but the main problem was that the tongue base did not adhere to the pharyngeal wall, resulting in inadequate pressure formation to propel food into the esophagus. The tongue base, pharynx, and larynx, which can be directly affected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), were impacted, causing the tongue base and uvula to detach from the pharyngeal wall. However, as the condition of DM improved, the suprahyoid muscles, which are not directly affected by SARS-CoV-2, became stronger, leading to improved UES opening.
SARS-CoV-2 utilizes the membranous angiotensin-converting enzyme 2 receptors for cellular entry in humans. Subsequently, SARS-CoV-2 can induce innate and adaptive immune responses, commonly referred to as cytokine storms. The interferon (IFN) pathway is activated in various clinical subtypes of myositis, with type 1 IFN being significantly upregulated in patients with DM. Type 1 IFN, which plays a crucial role in muscle fiber damage in DM patients, is implicated in the organ damage observed in COVID-19 [7].
In this case report, the patient demonstrated improvement in muscle strength and normalization of CK levels with steroid treatment, but dysphagia did not improve. It is suggested that COVID-19 may hinder the recovery of dysphagia in steroid-responsive dermatomyositis patients.