INTRODUCTION
Tuberculosis of the central nervous system accounts for 5% of extra-pulmonary cases and it is seen most often in young children [1]. Although it has long been recognized to cause hypopituitarism, the prevalence of endocrine dysfunction is not known but has been reported to be low. To our knowledge, only four cases of pituitary dysfunction after tuberculous meningitis have been reported in Korea [2–4]. However, when the signs and symptoms are not obvious, hypopituitarism can be overlooked. In this report, we describe an unusual case of hypopituitarism and severe hypovitaminosis D presenting as osteoporotic fracture after cured tuberculous meningitis.
CASE REPORT
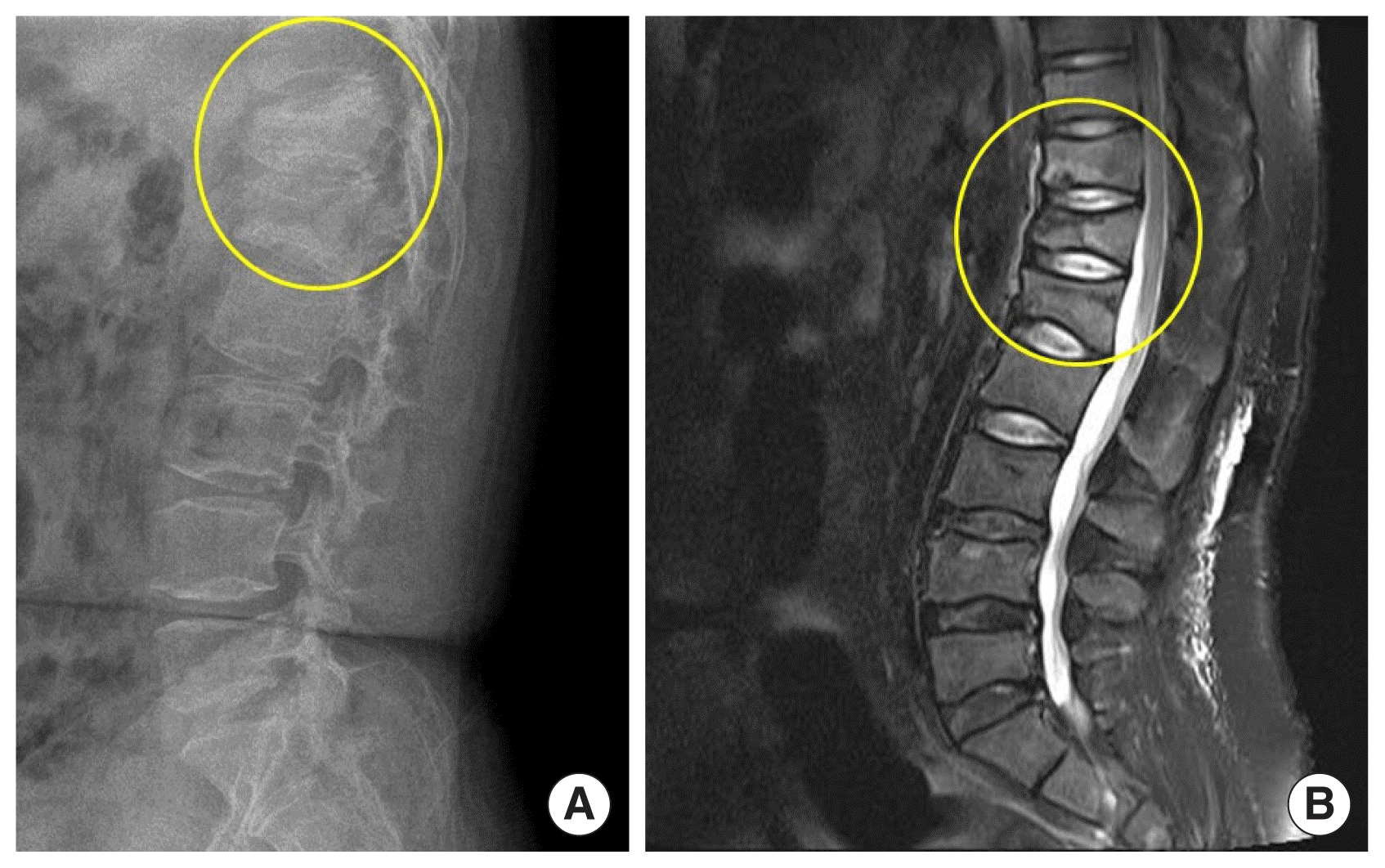
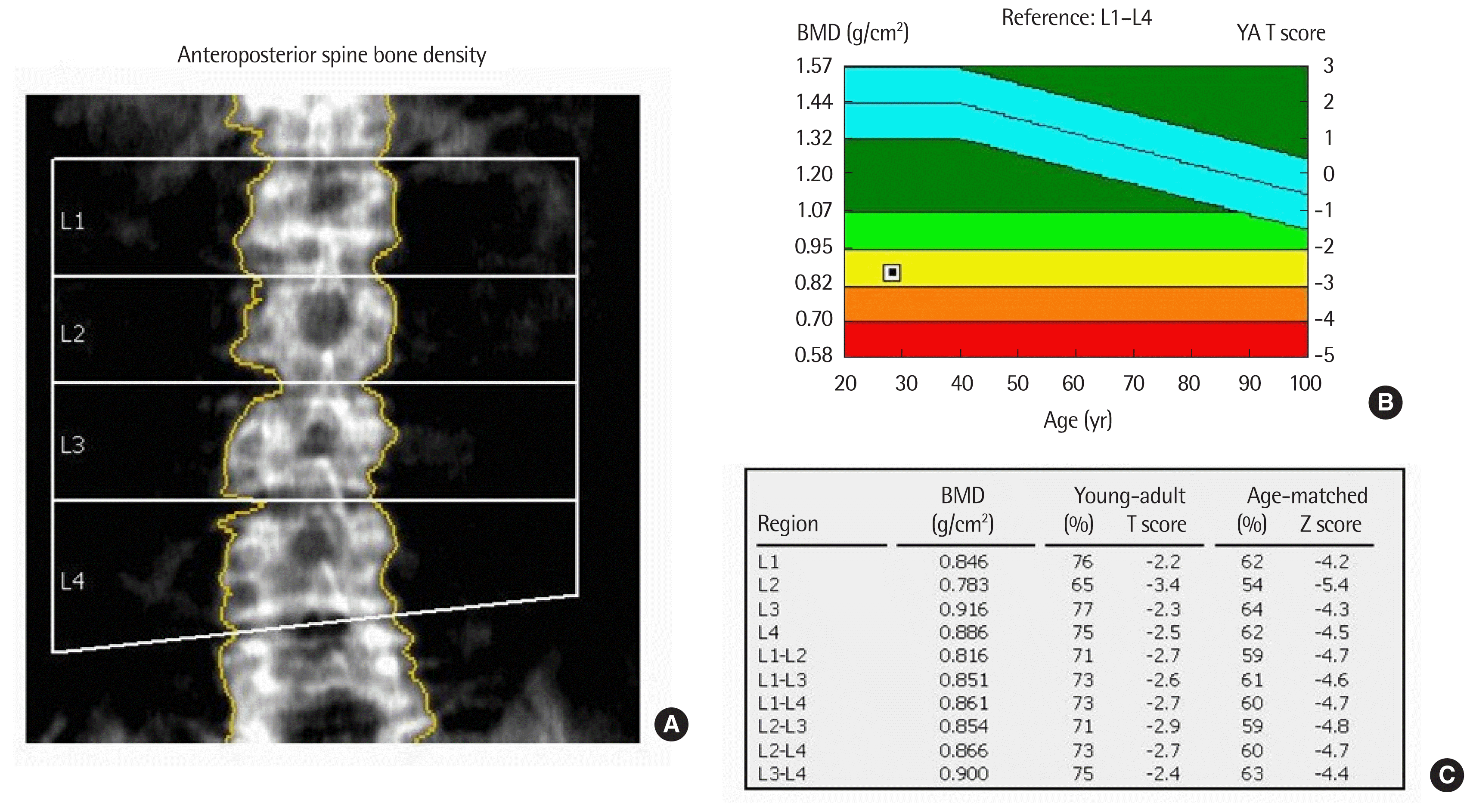
A 28-year-old male patient was referred to Chonbuk National University Hospital because of spontaneously developed back pain and incidentally detected immature leukocytes on blood test. He has a history of tuberculous meningitis at age 4 years that was cured after treatment with anti-tuberculosis medication. Physical examination revealed a small testis, short stature (height 154.2 cm), and morbid obesity (weight 93.5 kg, body mass index 39.3 kg/m2) without evidence of secondary sexual characteristics. After admission, his daily urine amount was >5 L and his urine amount did not decrease after water restriction. Blood tests revealed the following: white blood cell count, 7,510/μL; and 64% immature cells. The serum levels of hormones were as follows: follicle stimulating hormone <0.3 mIU/mL (normal range, 1.4 to 18.1 mIU/mL); luteinizing hormone <0.07 mIU/mL (normal range, 1.5 to 9.3 mIU/mL); testosterone 0.01 ng/mL (normal range, 2.36 to 9.96 ng/mL); free testosterone 0.01 pg/mL (normal range, 8.8 to 27 pg/mL); insulin-like growth factor (IGF)-1 8 ng/mL (normal range, 109 to 329 ng/mL); prolactin 3.8 ng/mL (normal range, 1.8 to 15.9 ng/mL); free thyroxine 14.60 pmol/L; thyroid stimulating hormone 4.88 μIU/mL (normal range, 0.55 to 4.78 μIU/mL); morning cortisol 6.5 μg/dL; basal adrenocorticotropic hormone 22.9 pg/mL; and maximal cortisol level after 250 μg cosyntropin stimulation 22.8 μg/dL. The serum 25(OH) vitamin D3 level of 8.1 ng/dL, parathyroid hormone of 133.16 pg/mL (normal range, 11 to 62 pg/mL), total calcium of 8.3 mg/dL (normal range, 8.4 to 10.2 mg/dL), and phosphorus of 5.1 mg/dL (normal range, 2.5 to 4.5 mg/dL) were also measured. Bone marrow aspiration and marrow biopsy were performed, and the results were compatible with acute lymphoblastic leukemia. In addition, the patient complained of persistent back pain. Lumbar-sacral radiography and magnetic resonance imaging (MRI) were performed and showed multiple compression fractures in the T11, T12, and lumbar spine 1 vertebral bodies (Fig. 1). The bone mineral density in lumbar spines was markedly decreased below the expected range for age (mean Z score=−4.7) (Fig. 2). Brain MRI showed no abnormal lesion but revealed focal encephalomalacic changes in the cerebral cortex. Acute leukemia needed urgent chemotherapy. Therefore, the patient received systemic chemotherapy soon after the diagnosis. Testosterone replacement and vitamin D replacement were started. Polyuria was improved after oral desmopressin administration.
DISCUSSION
Tuberculous meningitis is a well-known cause of hypothalamic pituitary dysfunction. However, deficiencies of anterior pituitary hormones may only become evident years after recovery because symptoms are of an insidious onset and sometimes nonspecific. One retrospective study showed that 20% of patients developed hypopituitarism after recovery from tuberculous meningitis in childhood [5]. In that study, growth hormone (GH) and gonadotropin deficiency were common problems as in our patient. GH and gonadotropin are essential for normal growth and sexual development in childhood, and for the maintenance of healthy body composition. It is also well known that the most common cause of secondary osteoporosis of the men is testosterone deficiency. Therefore, because early diagnosis and appropriate treatment can be of great benefit to these patients, clinicians should keep in mind the possibility of the development of hypopituitarism in their patients, and lifelong follow-up is needed. On the other hand, laboratory test of our patient showed secondary hyperparathyroidism with vitamin D deficiency. Hypovitaminosis D and hypopituitarism are strong risk factors of osteoporosis and osteoporotic fracture. Although there is limited evidence about the relation between hypovitaminosis D and hypopituitarism, some studies suggested that the incidence of vitamin D deficiency increases in the presence of hypopituitarism [6]. Furthermore, a positive association between vitamin D and the GH/IGF-1 axis has also been reported. Both GH and IGF-1 increase the production of vitamin D by cultured kidney cells, and another study revealed that vitamin D increases circulating IGF-1 in adults [7,8]. Therefore, it is reasonable to monitor the vitamin D levels in patients with hypopituitarism or GH deficiency. Furthermore, many studies have reported low serum concentrations of 25-hydroxyvitamin D to be associated with many non-skeletal disorders. Vitamin D deficiency is also considered a possible risk and poor prognostic factor of hematologic malignancies [9]. However, whether low vitamin D is the cause or the result of ill health is not established. Uncertainty also exists as to whether vitamin D supplementation alone can improve clinical outcomes. Further studies are needed to elucidate this issue. But vitamin D replacement may have a beneficial effect on the treatment of low vitamin D related hyperparathyroidism as our patient. In summary, the prevalence of hypothalamic-pituitary dysfunction after recovery from childhood tuberculous meningitis is probably much higher than has been previously recognized. In addition, it is well known that pituitary hormones are important for sexual development and healthy body composition. Therefore, proper diagnosis and replacement of pituitary hormone for such patients is essential for reducing morbidities.