INTRODUCTION
Atrial fibrillation (AF) is the most common chronic arrhythmia associated with heart failure (HF) [1–4]. Approximately half of patients with HF show preserved left ventricular ejection fraction (LVEF), and AF is more prevalent in patients with HF with preserved rather than reduced LVEF [5–9].
AF with left ventricular (LV) diastolic dysfunction plays an important role in the prevalence and severity of HF, rather than AF with systolic dysfunction. AF is also associated with increased mortality in patients with HF with preserved LVEF as well as reduced LVEF [10–12]. Therefore, evaluation of LV diastolic function is important in chronic AF patients with preserved LVEF. However, conventional echocardiographic methods used to assess diastolic dysfunction in sinus rhythm cannot be readily applied in AF because of a lack of atrial contraction due to asynchronicity and variable heart rate [4,13,14]. Several echocardiographic and invasive studies examining diastolic dysfunction in AF have used the ratio of trans-mitral early peak flow velocity (E) to tissue Doppler mitral annular motion velocity (e′), the ratio of E to color M-mode LV propagation velocity (Vp), deceleration time (DT), and pulmonary capillary wedge pressure (PCWP) by measurement devices [13–18]. However, the association between non-invasive echocardiographic estimation of LV filling pressure and invasive measurement of LV filling pressure remains unclear and is a challenging issue in chronic AF patients with preserved LVEF.
The purpose of this study was to investigate the correlation between non-invasive echocardiographic parameters and the invasive measurement of LV filling pressure and to identify which echocardiographic parameters can predict LV diastolic function in chronic AF patients with preserved LVEF.
MATERIALS AND METHODS
1. Patient population
The medical records of 90 consecutive patients who presented with chronic AF and preserved LVEF from January 2011 to September 2015 were evaluated retrospectively. All patients had their conditions confirmed on more than two consecutive electrocardiograms over a 3-month period. Preserved LVEF was defined as greater than 50% LVEF with no regional wall motion abnormalities evident on echocardiography. Patients were excluded from the study based on the following criteria: paroxysmal AF, rheumatic disease, prosthetic mitral valves, mitral stenosis, aortic valve stenosis, severe valvular insufficiency, pulmonary hypertension, or a history of pulmonary embolism.
The institutional review board of Soonchunhyang University Gumi Hospital approved the study protocol (IRB approval no., 2015-14). Written informed consent was obtained from all participating patients.
2. Echocardiography
Standard transthoracic two-dimensional, M-mode, and Doppler echocardiograms were performed in the left lateral decubitus position with GE Vivid 7 instrument (GE Healthcare, Milwaukee, WI, USA) equipped with M4S transducers within 24 hours before cardiac catheterization. Measurements were taken according to the recommendations of the American Society of Echocardiography [19]. We measured LV end-diastolic and end-systolic volumes and LVEF using the modified biplane Simpson’s method, and left atrial (LA) volume by the prolate ellipse method. E, e′, and Vp were measured using the dual Doppler imaging method. We calculated right atrial (RA) pressure from the inferior vena cava diameter and the presence of inspiratory collapse. Pulmonary artery systolic pressure (PASP) was determined from combining the tricuspid regurgitation gradient and calculated RA pressure in the absence of a gradient of pulmonary valve flow velocity [20]. The measurements reported are an average of five consecutive cardiac cycles. All echocardiographic measurements and estimations were performed without knowledge of invasive measurement of LV filling pressure.
3. Brain natriuretic peptide measurements
Blood samples were obtained for measurement of brain natriuretic peptide (BNP) within 24 hours of echocardiographic examination. BNP was measured by chemiluminescence enzyme immunoassay.
4. Hemodynamic measurements
Left heart catheterization was performed via radial or femoral approach. The measurement of LV end-diastolic pressure (LVEDP) was performed with a pig-tail catheter before coronary angiography. Measurements from 10 consecutive cardiac beats were recorded by an investigator unaware of the echocardiographic data and averaged to obtain a final measurement.
5. Statistical analysis
Data are presented as the mean±standard deviation. Comparisons between variables were performed using the t-test and Fischer’s exact test. Correlations between echocardiographic parameters and LVEDP were determined using linear regression analysis. The diagnostic accuracies of echocardiographic parameters were compared by receiver operating characteristic (ROC) curve analysis. Statistical significance was defined as P<0.05. All statistical analyses were performed using the IBM SPSS ver. 20.0 software package (IBM Corp., Armonk, NY, USA).
RESULTS
1. Baseline and echocardiographic characteristics
The patients were divided into two groups: group 1 included 52 patients with normal LVEDP and group 2 included 38 patients with increased LVEDP (>15 mm Hg). The baseline characteristics of the patients are presented in Table 1. The mean age of the patients was 68.8±10.1 years, and 47 patients were male. Eighteen patients had New York Heart Association class 3 or 4 of HF, 33 had coronary artery disease or prior myocardial infarction, and 26 patients had only AF. There were no significant differences between the two groups in age, sex, medical history, or drug history. The echocardiographic parameters, hemodynamic measurements, and laboratory data are presented in Table 2. The mean LVEF of all patients was 60.9±5.2, and the mean heart rate was 82.2±22.4 beats/min. LVEF was preserved, and there were no significant differences between the two groups. Heart rate was well controlled in both groups. The mean LVEDP was 14.9±4.5 mm Hg and the mean minimal LV pressure was 10.6±4.7 mm Hg. The LA volume index, mitral E, PASP, E/e′, and E/Vp were significantly higher and the Vp was significantly lower in group 2 than in group 1 (LVEDP >15 mm Hg). However, there were no significant differences between the two groups in terms of EF, DT, HR, or BNP.
2. Relationship between left ventricular filling pressure and echocardiographic parameters
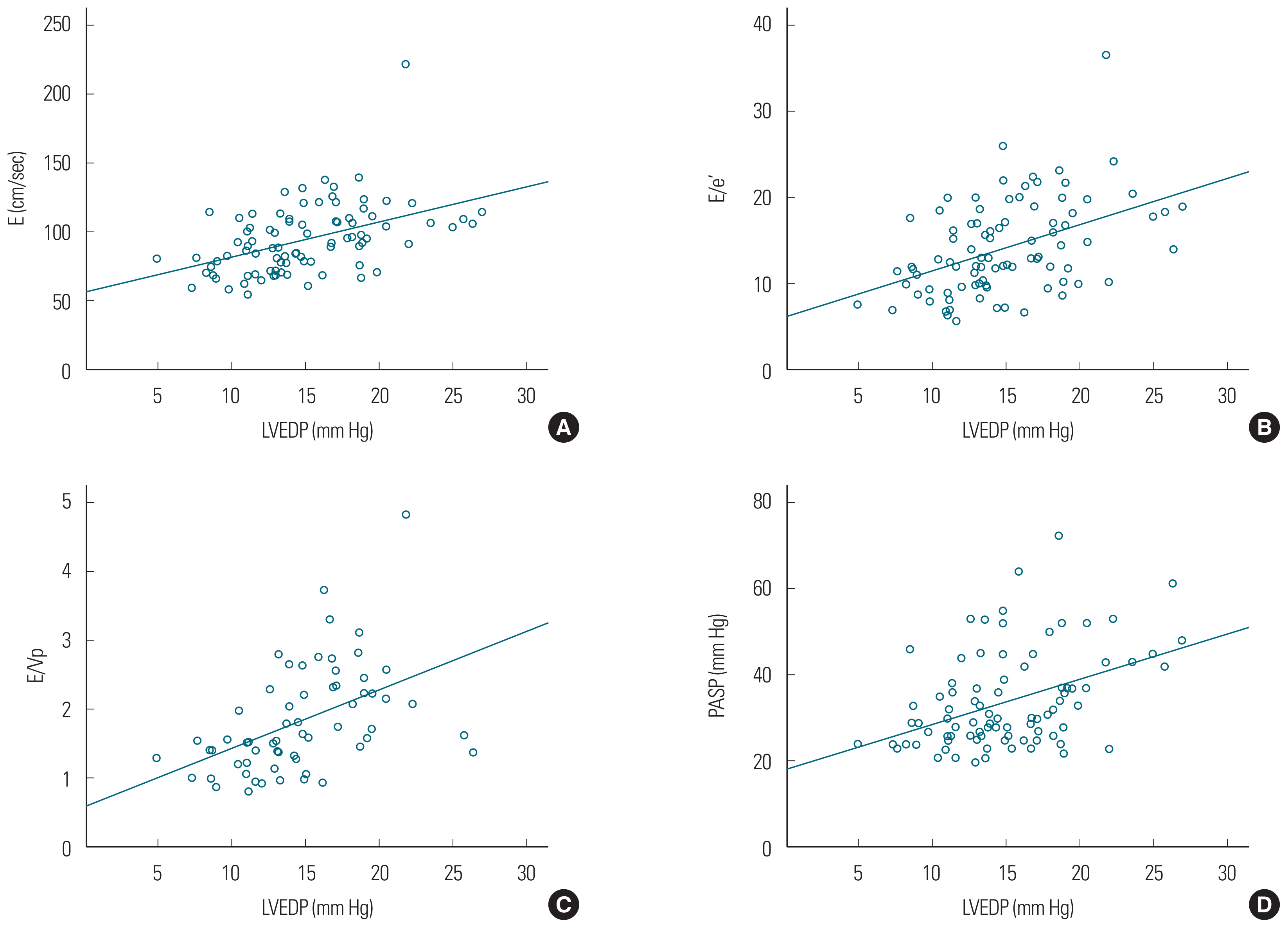
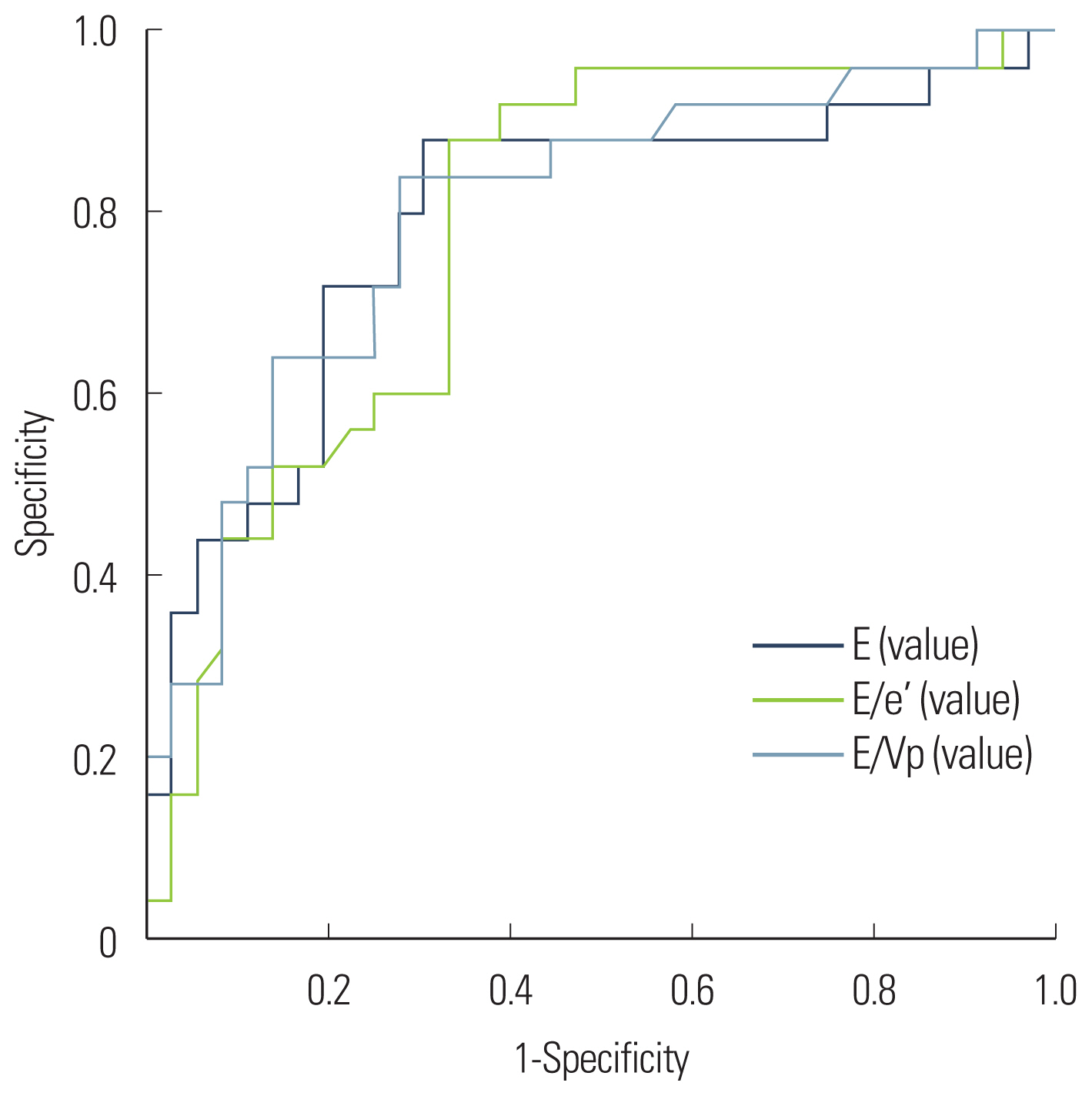
E (r=0.463, P<0.001), E/e′ (r=0.449, P<0.001), and E/Vp (r=0.471, P<0.001) demonstrated significant linear correlations with LVEDP. The PASP (r=0.422, P<0.001) and LA volume index (r=0.313, P=0.003) values were positively correlated with LVEDP (Fig. 1). Also, E (r=0.267, P=0.011), E/e′ (r=0.262, P= 0.013), E/Vp (r=0.325, P=0.011), PASP (r=0.289, P=0.006), and LA volume index (r=0.247, P=0.019) demonstrated positive linear correlations with minimal LV pressure (Fig. 2). The predictive values of E, E/e′, and E/Vp for the detection of LVEDP >15 mm Hg were determined with ROC curve analysis (Fig. 3) using the area under the curve (E, 0.782; E/e′, 0.790; E/Vp, 0.799). The optimal cutoff value of E/e′ was 13 (sensitivity, 88%; specificity, 67%) to predict >15 mm Hg LVEDP. E >90 cm/sec predicted elevated LVEDP (>15 mm Hg) with a sensitivity of 84% and a specificity of 70%. Also, E/Vp >1.6 predicted elevated LVEDP (>15 mm Hg) with a sensitivity of 80% and a specificity of 72% (Fig. 3).
DISCUSSION
Diastolic dysfunction plays an important role in the development of e related with AF [3,4]. Patients HF with preserved LVEF comprise approximately 40%–50% of the total HF population, and AF is more likely to occur in these patients [5–9]. AF was associated with reduced survival in patients with HF with preserved LVEF [10–12]. Therefore, evaluation of LV diastolic function is important in chronic AF patients with preserved LVEF to prevent worsening of HF. Until recently, several studies have evaluated the relationship between diastolic dysfunction and echocardiographic parameters in the setting of AF [13–18]. However, conventional echocardiographic methods used to assess diastolic dysfunction in sinus rhythm cannot be readily applied in AF because of the lack of atrial contraction due to atrial contractile asynchronicity and variability in heart rate [4,13,14]. The aim of this study was to investigate echocardiographic parameters for predicting LV filling pressure and diastolic function in chronic AF patients with preserved LVEF.
In the present study, the main findings were: (1) LVEDP was elevated in chronic, persistent AF patients with preserved LV systolic function, and (2) E, E/e′, and E/Vp had significant predictive value for LVEDP in chronic AF patients with preserved LVEF.
1. Elevated left ventricular filling pressure in chronic atrial fibrillation patients
In AF, atrioventricular asynchrony and irregular ventricular response can adversely affect ventricular function, leading to a decrease in cardiac output and impairment of diastolic filling [1,4,10,13]. Moreover, in chronic AF, these characteristics can result in fluid retention and LVEDP elevation. In the current study, we demonstrated the elevation of LVEDP by invasive measurement of LV filling pressure (14.9±4.5 mm Hg), which showed the impact of AF on diastolic dysfunction in chronic AF patients with preserved LVEF.
2. The relationship between echocardiographic parameters and left ventricular filling pressure
The E velocity is the early LV filling velocity and is influenced by LV relaxation and LA pressure [13,15]. Several studies have demonstrated weak to moderate correlations between peak E and LV filling pressure via measurement of PCWP in patients with AF, but its predictive value was not obvious [13,16,17]. In our study, modest correlations were found between E and LVEDP, as well as minimal LV pressure, by direct measurement of LV filling pressure; here, E >90 cm/sec was the predictive value for detection of elevated LVEDP (>15 mm Hg). Peak E velocity is associated either with normal LV relaxation and low LV filling pressure or abnormal LV relaxation and elevated LA pressure [13,15]. Our results seemed to reflect the latter condition.
Prior studies on the E/Vp ratio in patients with AF demonstrated a modest correlation with LV filling pressure, and presented a predictive value associated with increased LVEDP [13,21]. A previous study demonstrated that E/VP >1.4 predicted an elevated PCWP (≥15 mm Hg) with a sensitivity of 72% and a specificity of 100% [13]; another study found that mean E/Vp ≥1.7 predicted increased BNP (≥200 pg/mL) with a sensitivity of 80% and a specificity of 84% [21]. Our results were similar to those of the above-mentioned studies and demonstrated that E/Vp is modestly correlated with LV filling pressure and has good predictive value (>1.6) in the detection of elevated LVEDP (>15 mm Hg), but we did not find a relationship between E/Vp and BNP. A rapid decrease in Vp, as well as an increase in peak E velocity, was associated with elevated LVEDP and impaired LV relaxation [22]. So, E/Vp increases with HF deterioration and is a more sensitive measurement than is E alone. However, in our study, the predictive value of E and E/Vp was similar in patients with preserved LVEF. So, we posit that our study population consisted of patients with less advanced HF. These characteristics may influence the relationship between E/Vp and BNP, and the predictive value of E and E/Vp.
Theoretically, E/e′ is the preferred measure in patients with preserved LVEF because it is less variable than E/Vp [23]. In a previous study, E/e′ was shown to be a reasonably sensitive and easy to measure parameter for the prediction of diastolic dysfunction in patients with AF; E/e′ ≥11 predicted elevated LV filling pressure (≥15 mm Hg) with a sensitivity of 75% and a specificity of 93% [14]. However, that study had a small number of patients with AF and variable characteristics that could affect the value of E/e′ for predicting elevated LVEDP. We used a larger study population that consisted of chronic AF patients with preserved LVEF and direct measurements of LV filling pressure. Our study demonstrated a good correlation between E/e′ and LVEDP, as well as minimal LV pressure, and shows the clinical usefulness of E/e′ in predicting elevated LV filling pressure. Recently, some studies reported that E/e′ recorded using dual Doppler echocardiography had a good correlation and predictive value for PCWP and BNP [24,25]. Other studies suggest that lateral mitral E/e′ is more highly correlated with PCWP in patients with preserved LVEF [23,26].
Our study showed that the left atrial volume index (LAVI) and PASP had weak correlations and no predictive value for LV filling pressure in chronic AF patients with preserved LVEF. LA enlargement occurs frequently in AF, and the LAVI is influenced by LV filling pressure. However, some previous studies also observed no relationship between LAVI and LV filling pressure and did not find clinical usefulness of this measurement for estimating LV filling pressure [27–29]. Several studies have demonstrated a relationship between increased PASP and LV diastolic dysfunction in patients with myocardial infarction or preserved LV systolic function with the sinus rhythm [30,31]. Furthermore, Neuman et al. [32] showed a good correlation between PASP and LVEDP in patients with chronic AF. However, in our study that consisted of chronic AF patients with ‘preserved EF’, PASP was observed to be only weakly correlated with LVEDP. We found that LVEDP had a weak impact on PASP, when LVEF was preserved, unlike what has been reported in previous studies.
Echocardiographic evaluations of diastolic dysfunction are difficult and often unclear in patients with AF because the characteristics of AF have not been extensively studied using direct measurement of LV filling pressure. In our study, we examined the elevation of LVEDP by direct measurement of LV filling pressure and demonstrated the relationship and predictive value of simple volume-dependent echocardiographic parameters for LV filling pressure in chronic AF patients with preserved LVEF.
3. Limitations
Our study had a few limitations. First, we obtained data on LV filling pressure from a small number of patients only. The patients were elderly, had controlled heart rates, and their population was composed of a relatively homogeneous and unrepresentative cohort of chronic AF patients with preserved LVEF. Second, echocardiographic measurements and LV catheterization were performed at different times in the patients, which could impact the correlation between echocardiographic parameters and LV filling pressure. Therefore, further investigation into the prognostic value and serial changes in echocardiographic parameters is warranted.
4. Conclusions
In our study, we observed that E, E/Vp, and E/e′ were well correlated with LV filling pressure in patients with chronic AF and preserved LVEF. Moreover, our findings demonstrated the usefulness of E, E/Vp, and E/e′ for estimating LV filling pressure in these patients. E >90 cm/sec, E/Vp >1.6, and E/e′ >13 were suggestive of elevated LVEDP (>15 mm Hg) in patients with chronic AF with preserved LVEF.