INTRODUCTION
Acquired cystic kidney disease (ACKD) is a common complication in patients with end-stage renal disease (ESRD) undergoing dialysis. ACKD could be strongly suspected if no evidence of familial cystic kidney disease is observed and if kidney function decreases. For a diagnosis of ACKD, more than three kidney cysts must to be detected by using ultrasonography (US) or abdominal computed tomography (CT) [1]. Similarly, patients with autosomal dominant polycystic kidney disease (ADPKD) develop multiple renal cysts as they age. ADPKD is caused by genetic mutations; however, approximately twenty five percent of patients have no known family history of ADPKD [2]. Therefore, it may be difficult to distinguish ACKD and ADPKD. Making a differential diagnosis is important, as the risk of renal cell carcinoma (RCC) is much higher in patients with ACKD than in those with ADPKD [3]. Herein, we present a case of ACKD resembling ADPKD that progressed to RCC in a patient with sclerosing encapsulating peritonitis (SEP) who had been receiving continuous ambulatory peritoneal dialysis (CAPD) for 15 years.
CASE REPORT
A 40-year-old man presented to the emergency clinic at Inha University Hospital with symptoms of abdominal pain, nausea and vomiting for three days. He had been diagnosed with hypertensive chronic kidney disease at the age of 24 years. His disease progressed to ESRD, and he began undergoing CAPD at the age of 25 years. He had been admitted three times during the 15 years of undergoing CAPD for recurrent peritonitis.
The dialysis modality was changed from CAPD to hemodialysis at the age of 39 years, because of ultrafiltration failure and recurrent abdominal pain caused by SEP.
He had no family history of kidney disease; no known anatomical abnormalities were noted by using abdominal US performed when he was in his twenties. He intermittently complained of abdominal pain and dyspepsia, both of which started 3 months previously. His abdomen was severely distended and increased bowel sound was noted. Physical examination also revealed fluid shifting.
Blood tests revealed a white blood cell count of 10.3×103/μL, hemoglobin level of 11.6 g/dL, platelet count of 626×103/μL, erythrocyte sedimentation rate of 52 mm/hr, and a C-reactive protein level of 7.44 mg/dL.
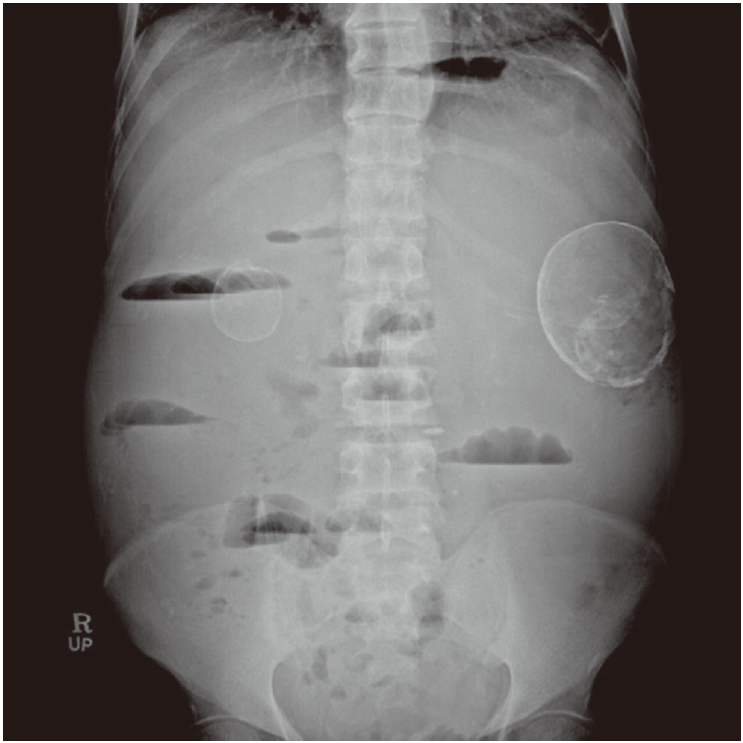
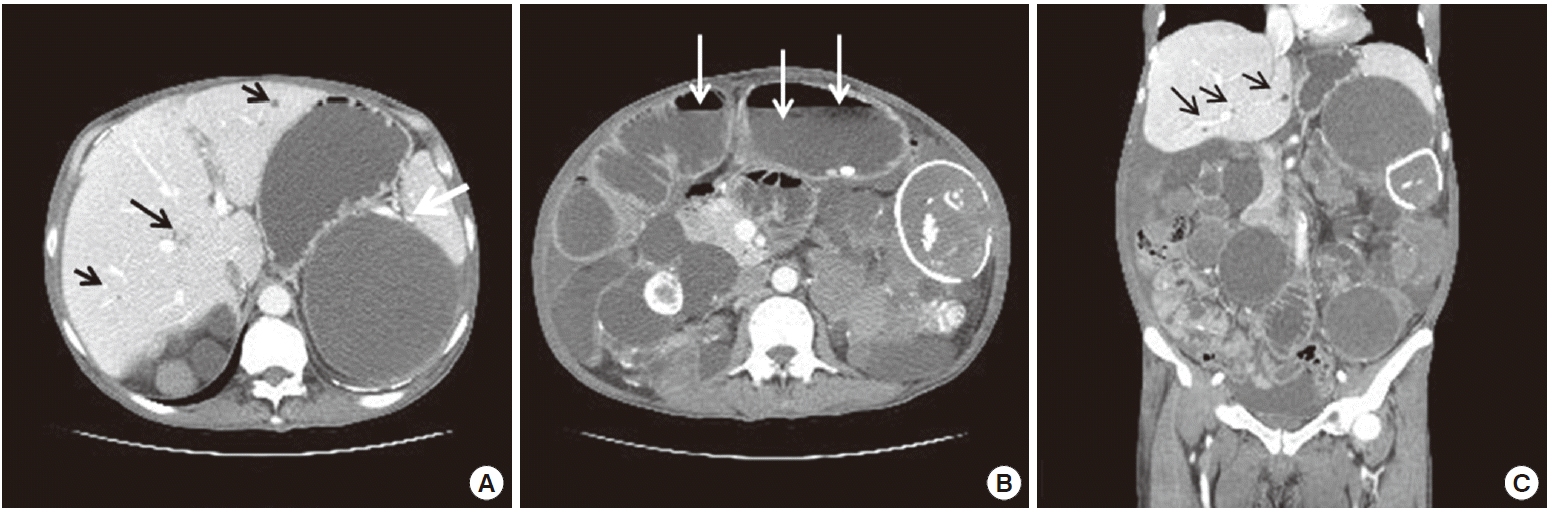
Paracentesis was performed, and ascitic fluid analysis revealed lymphocyte-rich exudates with a serum ascitic albumin gradient of 0.9 g/dL, ascitic fluid red blood cell count of 1,420/mm3, white blood cell count of 1,420/mm3 (polymorphonuclear neutrophil leukocytes, 3%; lymphocytes, 65%), protein concentration of 4.9 g/dL, adenosine deaminase level of 41.3 IU/L, and carcinoembryonic antigen level of 0.66 ng/mL. An abdominal radiograph revealed a step ladder sign and two eggshell calcified cysts (Fig. 1). Abdominal CT was performed to evaluate possible bowel obstruction, and it revealed two calcified cystic lesions. There were findings suggestive of ileal obstruction caused by peritoneal adhesion, as well as multiple bowel calcifications and multiple cysts in both kidneys, of which the wall showed calcification with ascites. Multiple hepatic cysts were also observed (Fig. 2).
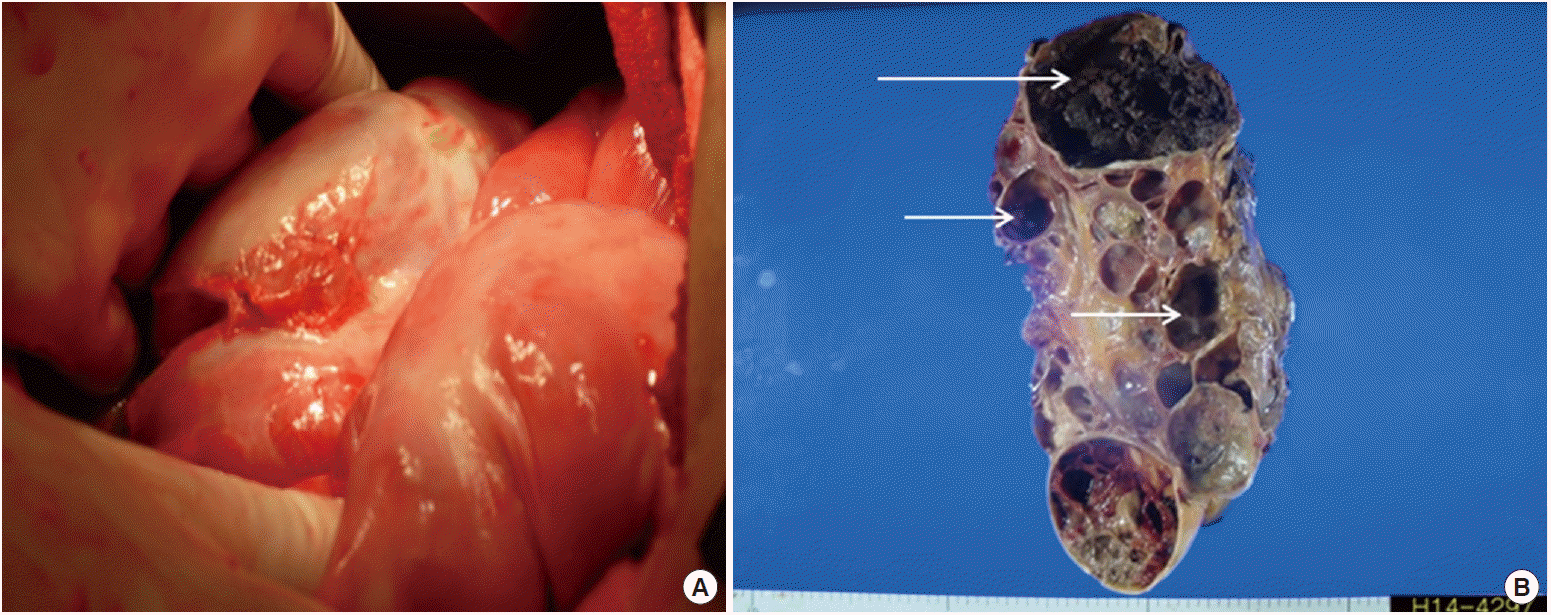
Left radical nephrectomy was performed on the 10th day after admission. During the surgery, examination of the intestine revealed that the small bowel was covered with a thick fibrous membrane with severe adhesions (Fig. 3A). Motility of the organ significantly decreased. These findings are consistent with that of SEP.
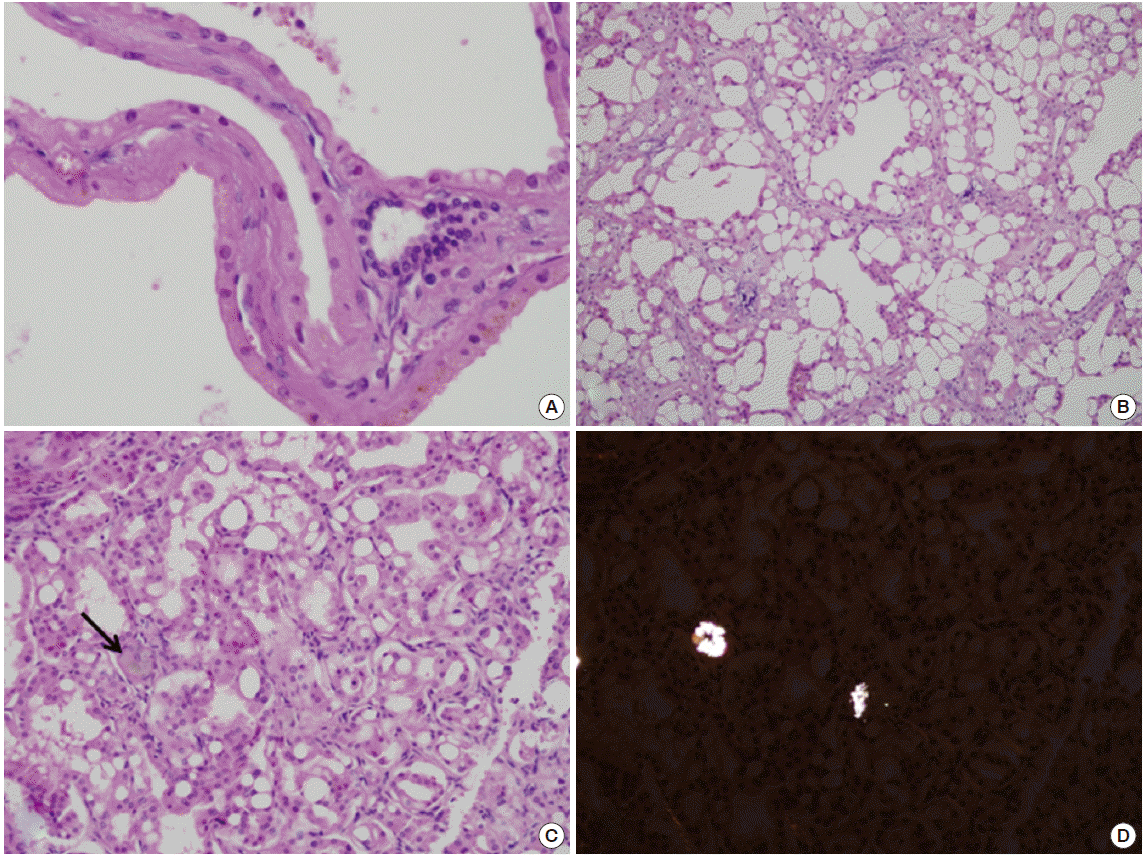
The size of the resected kidney was 28×17×12 cm, with a weight of 2,300 g. The kidney had a multilocular polypoid appearance. Gross sectioning showed multiloculated cysts, ranging from 1 cm to 11 cm in diameter (Fig. 3B). Microscopic examination revealed that the cysts were lined by large cuboidal cells with abundant eosinophilic cytoplasm (Fig. 4A); normal renal parenchyma was rarely observed. The tumor areas were multifocally scattered with the largest measuring 2×1.5 cm. The tumor cells contained abundant eosinophilic cytoplasm and prominent nucleoli. Inter- or intra-cytoplasmic microscopic lumina were frequently observed, forming a sieve-like appearance (Fig. 4B). Oxalate crystals were occasionally observed within the tumors (Fig. 4C, D). The tumor cells were positive for a-methylacyl-coenzyme A racemase (AMACR) and CD 10, but negative for cytokeratin 7. The tumors were identified as ACKD-associated RCC, confined to the kidney with a pathologic stage of TIa. Distant metastases were not observed.
Therefore, no additional treatment was administered for the RCC. We initiated methylprednisolone at a dose of 48 mg/day for SEP. He was discharged upon symptom resolution on the 30th day after admission. The patient was administered a tapered dose of methylprednisolone for 6 months after hospital discharge.
DISCUSSION
ACKD is a common complication in patients undergoing dialysis treatment. It should be strongly suspected if there is no history of familial cystic kidney disease and if kidney function decreases. The prevalence of ACKD increases with longer durations of dialysis [1]. ACKD occurs in approximately ninety percent of dialysis patients who are undergoing dialysis for more than 5 years [4]. Although controversies still exist, there is no difference between hemodialysis and peritoneal dialysis in influencing incidence of ACKD [4]. ACKD often presents with no symptoms, but sometimes can manifest as flank pain and hemorrhage. Additionally, ACKD progress to RCC from three to six percent of patients [1].
For a diagnosis of ACKD, at least more than three kidney cysts must be detected by using US, CT, or magnetic resonance imaging without known renal cysts before the initiation of dialysis [1]. Pathological diagnosis can be made if the cystic lesion involves more than 25% of the renal parenchyma [1]. Usually in ACKD, the kidneys are small or normal sized and the diameters of renal cysts are less than 0.5 cm [1].
In ADPKD, however, both kidneys are enlarged and the sizes of the cysts are variable and grow as the age of patients increase. Extrarenal manifestations are common in ADPKD, including hepatic cysts (80-90%), seminal vesicle cysts (40%), mitral valve prolapse (25%), abdominal hernia (10%), pancreatic cyst (9%), and cerebral aneurysm (8%) [2]. RCC appeared in less than 1% of patients with ADPKD, and the incidence of RCC in patients with ADPKD is the same as that found in the general population. Commonly known as a genetic disease, ADPKD is caused by genetic mutations in the PKD1 and PKD2 genes.
However, approximately twenty five percent of patients have no known family history [2]. Therefore, making a differential diagnosis between ACKD and ADPKD is difficult. In our case, both kidneys were enlarged in the patient, with variable-sized cysts and multiple hepatic cysts observed. These findings are compatible with ADPKD. However, he had no family history of ADPKD, and no visible kidney cysts were detected by using US previously, which is more suggestive of ACKD. Genetic testing was not performed because of financial obstacles. Pathologic findings consistent with ACKD include several birefringent calcium oxalate crystals observed by using polarizing microscopy and the presence of inter- or intracellular lumina of the eosinophilic tumor cells by using light microscopy [5]. Immunohistochemical staining results are also compatible with ACKD, with positive results for AMACR and CD 10, which is a relatively specific feature of ACKD associated RCC different from other RCCs [5,6]. There are several known subtypes of RCC in patients with ESRD, including clear cell, papillary, chromophobe. However, the following two new subtypes of RCC have recently been shown to be associated with ESRD: ACKD-associated RCC and clear cell papillary RCC. The ACKD-associated RCC occurs only in patients with ESRD having ACKD, while clear cell papillary RCC can occur in patients with ESRD without ACKD [6].
Although less than ten percent of patients with ACKD proceed to malignancy, these patients have a 100-fold greater risk for the development of RCC than the general population [7]. ACKD-associated RCC tumors are often limited to the kidney and are smaller in size than other RCC tumors [6]. However, there have been a few reported cases of RCC with metastases to other organs or aggressive behaviors [8].
Old age, male gender, large kidney cysts, increased kidney sizes, and huge calcifications are known risk factors for malignant transformation [9]. It has been reported that screening test could reduce the risk of cancer death, with survival benefit [10].
Therefore, screening tests are needed regularly. Although some controversies exist about the appropriate times and optimal imaging modality for detecting malignancy, it is recommended that annual screening tests should be performed in young patients who are undergoing dialysis with ACKD [10]. Both CT scan and US are useful modalities for screening [10].
We describe herein a case of progression of ACKD, which resembled ADPKD, to RCC in a patient with SEP who was undergoing dialysis for 15 years. Many physicians focus on ADPKD rather than ACKD when there are enlarged kidneys and large cysts on diagnostic images, although RCC rarely occurs in ADPKD. Moreover, some physicians tend to focus on SEP rather than ACKD when the patient has been undergoing CAPD for a long period of time. This can lead to undetected malignancies. This case reminds us of the importance of differentiating ACKD from ADPKD and performing routine screening tests for ACKD and RCC in patients undergoing CAPD with SEP.