INTRODUCTION
The dual proliferation of the mesothelial cells and histiocytes is a pathologically rare condition. After it was first reported as nodular histiocytic/mesothelial hyperplasia (NHMH) by Rosai and Dehner [1] in 1975, 21 cases have been reported so far [2]. It is clinically worthy to mention that it can mimic malignancy and can be related to subsequent over-treatment. Although its etiology has not been fully understood, it is generally thought to be associated with repeated mechanical or chemical stimulation (inflammation, surgery, etc.) [3].
The previous reports mainly consist of cases with a nodular form of histiocytic/mesothelial hyperplasia. Herein, we first report a case of papillary histiocytic and mesothelial hyperplasia of the ovary which was found in the course of high-intensity focused ultrasound (HIFU) treatment for uterine adenomyosis and leiomyoma.
CASE REPORT
A 48-year-old female was referred to the hospital for further treatment of deep vein thrombosis (DVT) induced by diffuse enlargement of the uterus. She visited an outside hospital with groin pain 6 months ago and underwent inferior vena cava filter placement along with HIFU treatment 7 times for the uterus under the impression of DVT and diffuse enlargement of the uterus with adenomyosis and leiomyoma.
The laboratory findings of the first visit to Soonchunhyang University Seoul Hospital showed mild anemia (hemoglobin, 10.3 g/dL [reference, 12–16 g/dL] and hematocrit, 32.8% [reference, 36%–48%]). Coagulation tests showed a mild increase in prothrombin time (PT, 13.0 seconds [reference, 9.3–11.6 seconds]) and activated partial thromboplastin time (APTT, 31.3 seconds [reference, 27.8–41.7 seconds]), suggesting the effect of previous anti-thrombotic therapy. The liver function test was within the normal range (aspartate aminotransferase, 26 U/L [reference, 0–40 U/L] and alanine aminotransferase, 19 U/L [reference, 0–41 U/L]).
Dynamic contrast-enhanced pelvic magnetic resonance imaging (MRI) images revealed a diffusely enlarged uterus with adenomyosis and intramural and subserosal leiomyoma. The right ovary was posteriorly shifted by the levo-rotated uterus which was asymmetrically enlarged and had an enhancing soft tissue lesion surrounding the ovary. It should radiologically differentiate tumorous and non-tumorous diseases. Comparing the MRI findings of the outside hospital, the uterus showed heterogeneously hypointensity on T2-weighted images, hyperintensity on T1-weighted images, and no contrast enhancement. These findings were suggestive of HIFU-induced degenerative changes. But the right ovary showed no remarkable changes after HIFU treatment (Fig. 1). Under the impression of uterine adenomyosis with leiomyoma and right ovarian mass, total laparoscopic hysterectomy with right salpingo-oophorectomy and left salpingectomy was performed.
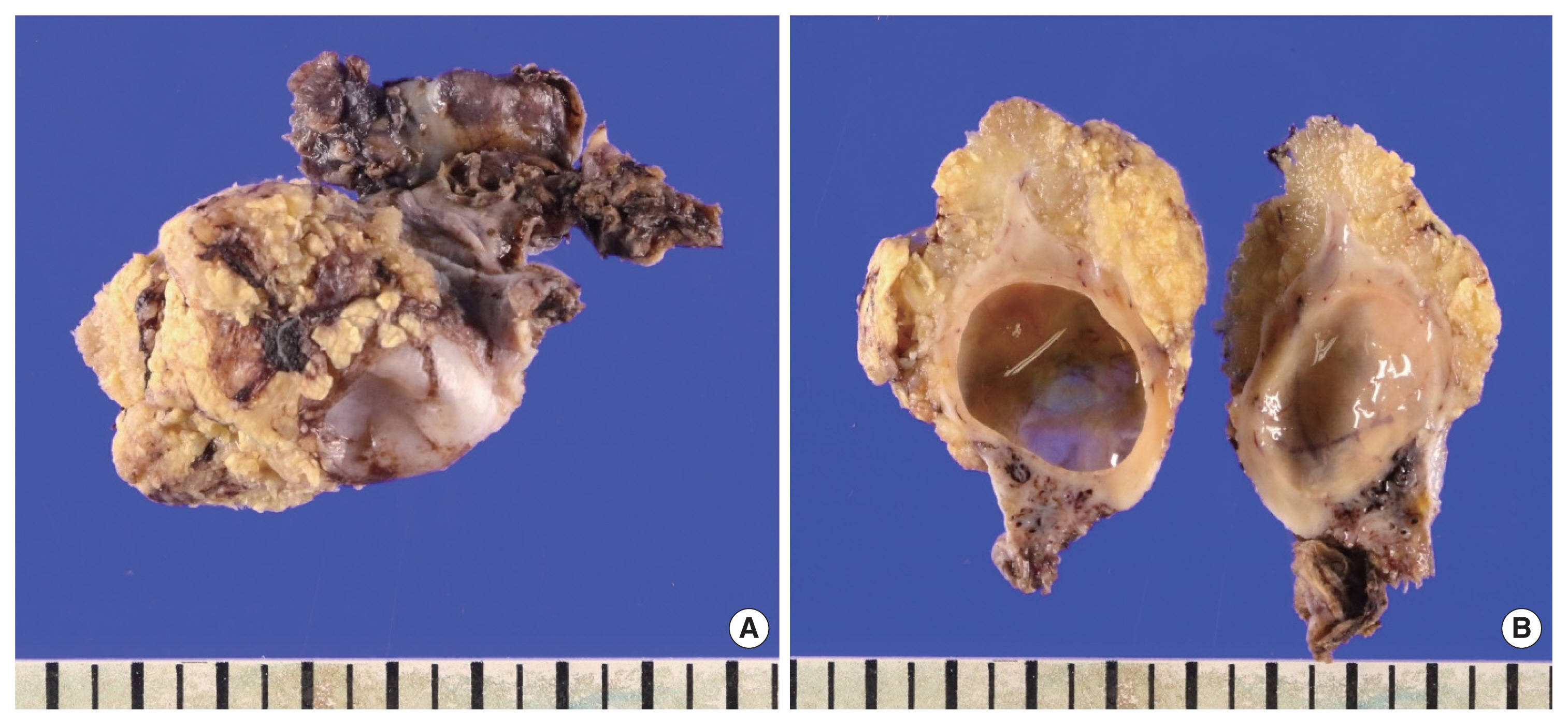
Macroscopically, the uterus weighed 750 g and measured 23×22×6 cm in aggregates of the resected fragments, containing multiple myoma masses and abundant myometrium with blood-tinged tiny cysts and focal necrotic changes; These histologic features were suggestive of HIFU induced thermal injury, correlated with MRI findings. The right ovary weighed 31 g and measured 5×4.5×3 cm. It showed a yellowish-gray mass-like growing peritoneal surface with papillary features. On the cut section, there was a thickened papillary lesion on the ovarian surface with an unrelated simple small cyst on the parenchyma (Fig. 2).
Microscopically, the right ovarian lesion showed papillary growth on the serosal surface (Fig. 3A). It had thick or thin papillary cores containing a marked aggregation of macrophages and thick-walled blood vessels on the sclerotic background of papillary cores (Fig. 3B, C). The mesothelial lining was single-layered and showed no remarkable cellular atypia. The immunohistochemical findings showed positive mesothelial markers (calretinin, mesothelin, and Hector Battifora mesothelial-1) in the lining mesothelial cells (Fig. 3E). The infiltrated macrophages were positive for CD68. The mesothelial cells were negative for p53, and there was no increase in the Ki-67 proliferation index or estrogen receptor positivity (Fig. 3F–H). The uterine myometrium revealed marked adenomyosis and leiomyoma, and some myomas had infarctions presumed to be caused by HIFU treatment (Fig. 3I).
The patient was confirmedly diagnosed with adenomyosis and leiomyoma of the uterus and nodular histiocytic and mesothelial hyperplasia with the papillary growth pattern of the right ovary. She was discharged on the third postoperative day without any complications. She was on follow-up for 6 months without any problems related to hysterectomy or DVT. The patient provided written informed consent for the publication of clinical details and images.
DISCUSSION
After the first description of NHMH in 1975 by Rosai and Dehner [1], 21 cases of the dual proliferation of histiocytes and mesothelial cells have been reported in the pericardium, pleura, peritoneum, and pelvis. However, they were all nodular varieties and papillary growing histiocytic and mesothelial hyperplasia have not been reported to the best of our knowledge.
HIFU is a newly emerging treatment modality used for solid tumors by integrating energy into the local area using a 0.8–3.5 MHz wave which generates heat causing cell necrosis at the target point. However, it is known to induce inadvertent injury to the hollow viscera adjacent to the target tumor [4]. Therefore, because of the past history of HIFU treatment to the uterus, we supposed that the previous HIFU treatment might have induced reactive changes in the right ovarian lesion. However, it was not confirmed by the subsequent radiological review of both the previous and present findings of pelvic MRI which showed no remarkable radiological changes in the right ovary.
The described histological findings, such as sclerosis of the stroma, thick-walled vessels, and infiltration of the reactive inflammatory cells can be frequently present in association with thermal or radiation injury [5,6]. To the best of our knowledge, these microscopic descriptions cannot be found in the previously reported cases of NHMH cases in these lesions [3,7,8]. Furthermore, vascular or stromal changes and an increase in histiocytic infiltration are frequently presenting pathological features of thermal or radiation injury to hollow viscus. In this respect, the possibility that the thermal injury induced by HIFU has added to the reactive histological changes could not be ruled out, although radiological findings indicated the pre-existing right ovarian lesion. We could not completely exclude the additional effects of HIFU inducing additional reactive vascular/stromal changes to the already existing papillary histiocytic and mesothelial hyperplasia of the right ovary.
Previous studies have focused on the comparative description of different characteristics between mesothelioma and histiocytic/mesothelial hyperplasia or between serous carcinoma of the ovary and histiocytic/mesothelial hyperplasia [3,7,8]. From a therapeutic point of view, it is more important to distinguish these diseases. Radiologically, MRI findings of the right ovarian lesion were unusual. There was an ovarian surface-like wall-thickening which was distinct from other typical ovarian malignancies. The possibility of the occurrence of mesothelioma in a single ovary is also less likely, if there were no suspicious mass-like lesions in the other peritoneal sites. The possibilities of typical ovarian malignancy or mesothelioma were clinico-radiologically less suggested. Further, papillary histiocytic and mesothelial hyperplasia could be hardly supposed due to its rare incidence. Histologically, the possibility of ovarian cancer was low in the present case considering that no invasion of the serous cells to the ovarian stroma was found (Fig. 3A) along with no serous cellular atypia and p53-negativity (Fig. 3D, F). The possibility of malignant mesothelioma was also low based on the findings of the mono-layered mesothelial cells showing no cellular abnormality, low Ki-67 proliferation index, and no history of asbestos exposures (Fig. 3G).
Hu et al. [9] proposed to unify mesothelial/monocytic incidental cardiac excrescence (MICE) and NHMH. Because MICE mainly presenting in patients undergoing cardiac catheterization show similar histological findings as those of NHMH, they insisted that NHMH is a similar entity to that of MICE and NHMH is a better choice. They have also explained the pathogenesis of MICE by two theories: the “reactive” theory describing that the mesothelial cells “gain” access to the intravascular compartment, and the “artifact theory” mentioning that the mesothelial cell migration through the cardiac wall is induced by cardiac surgeons during cardiac catheterization. However, this is quite different from the previous theory on the development of NHMH. Based on the previous etiologies, NHMH has been thought to have resulted from irritation by inflammation, mechanical trauma, or an adjacent tumor [7].
In the review of NHMH by Chung et al. [2], 16 cases among the 21 cases with NHMH had confirmed underlying conditions, and seven cases were caused by the adjacent space-occupying lesions (39%). Suarez-Vilela and Izquierdo-Garcia [10] explained one of the factors for the aggregation of these reactive cells by mechanisms of adhesion between mesothelium and leucocytes. The mesothelial cells in the serous cavities have intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 on their microvilli which act as the points of adhesion for the inflammatory cells including the histiocytes that express the counter receptors lymphocyte function-associated antigen-1 and very late antigen-4 [10]. Therefore, these adhesion molecules and respective ligands presented in the inflammation of the serosal cavity seem to mediate the genesis of NHMH.
If the past history is negative for abdominal trauma or abdominal surgery, it can easily be presumed that the huge enlargement by adenomyosis and uterine leiomyoma provides continuous mechanical stimulation to the right ovary to induce inflammation, thereby providing a basis for the genesis of histiocytic/mesothelial hyperplasia. One study showed a similar case of NHMH which did not have a history of abdominal surgery, but the patient had large intramural leiomyoma and endometriosis as accompanying disease [2].
Although the initial diagnosis of HIFU-induced ovarian mass was confusing due to the absence of a radiological picture of the right ovary obtained at the previous hospital, histiocytic/mesothelial hyperplasia is thought to be induced by the enlargement of the uterus except for the possible histological changes by HIFU. In addition, previous reports have mentioned a nodular variety of histiocytic/mesothelial hyperplasia, whereas, a distinguished papillary growth was noted in the present case. Further research is needed to clarify whether these characteristic findings are related to HIFU treatment.
To conclude, this benign reactive inflammatory lesion of the mesothelium mimicking malignancy must be kept in mind to avoid unnecessary treatment. We hope that our case presentation which includes radiological, and macro- and microscopic findings of papillary histiocytic/mesothelial hyperplasia would be useful for clinicians, radiologists, and pathologists. Herein, we report the first case of nodular histiocytic and mesothelial hyperplasia with a papillary growth pattern of the ovary related to a huge uterus.