INTRODUCTION
Aortitis is the all-encompassing term ascribed to inflammation of the aorta. Aortic wall inflammation may be infectious or more commonly noninfectious. The most common causes of aortitis are the large-vessel vasculitis, such as giant cell arteritis (GCA) and Takayasu arteritis. Aortitis also is associated with systemic lupus erythematosus, rheumatoid arthritis, the HLA-B27-associated spondyloarthropathies, anti-neutrophilic cytoplasmic antibodies (ANCA)-associated vasculitides, and Behcet disease. Infectious causes include tuberculosis, syphilis, salmonella, and human immunodeficiency virus (HIV). It may occur in isolation or accompany idiopathic retroperitoneal fibrosis [1].
Renal vein thrombosis has numerous etiologies. It occurs most commonly in patients with nephrotic syndrome due to its hypercoagulable state. The excessive urinary protein loss is associated with decreased antithrombin III, a relative excess of fibrinogen, and changes in other clotting factors; all lead to a propensity to clot. Other less common causes include renal cell cancer, renal transplantation, Behcet syndrome, and antiphospholipid antibody syndrome [2].
Isolated idiopathic aortitis presented with renal vein thrombosis is extremely rare and has not been reported in Korea yet. In this case report, we present a case of isolated idiopathic aortitis of the abdominal aorta presenting as left renal vein thrombosis due to compression by the thickened abdominal aorta wall, which improved after steroid therapy.
CASE REPORT
A 38-year-old man presented to an emergency department with abrupt left flank pain. He had a history of pneumonia recovered after antibiotic treatment and was a social smoker. He did not have any other symptoms or past medical history. Vital signs were stable, and physical examination revealed left costovertebral angle tenderness. Laboratory data showed white blood cell 16,060/µL, hemoglobin 17.5 g/dL, platelet 159,000/µL, creatinine 1.18 mg/dL, blood urea nitrogen 12 mg/dL, lactate dehydrogenase 411 IU/L, albumin 4.3 g/dL, total cholesterol 152 mg/dL, prothrombin time/activated partial thromboplastin time 13.4 sec/1.05, C-reactive protein 0.93 mg/dL, creatine kinase 270 IU/L, urinalysis protein 4+, red blood cell >1/2 per visual field. The urinary protein/creatinine ratio was 2,077 mg/g. Electrocardiogram was normal sinus rhythm without abnormality.
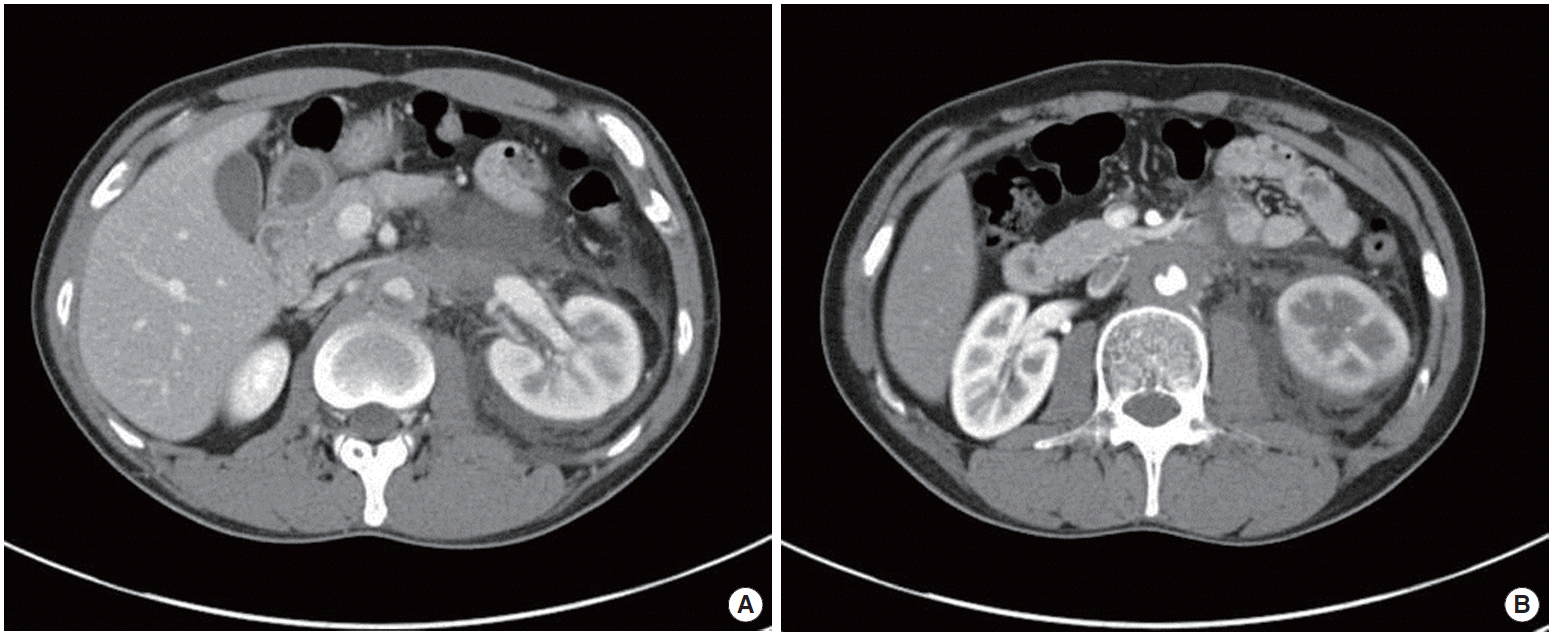
CT kidney enhancement with angiography was performed to exclude urinary tract stones and renal infarction. In the CT findings, the abdominal aortic wall was thickened and caliber of abdominal aorta was decreased with partial irregular thrombus (Fig. 1). It looked like the thickened abdominal aorta wall compressed the left renal vein. It caused left renal vein thrombosis, involving a length of about 4.7 cm, with decreased renal perfusion. There was no ureteric obstruction or hydronephrosis.
Additional investigations were performed and revealed serum immunoglobulin G (IgG) 1,220 mg/dL and IgG4 49 mg/dL, which was in the normal range. Other laboratory data was protein C activity 74%, protein S activity 88%, ANCA negative, antinuclear antibodies negative, anti-double strand-DNA antibody 2.96, anti-streptolysin O 7 IU/mL, immune fixation electrophoresis (serum) negative, immune fixation electrophoresis (urine) negative, anti-glomerular basement membrane antibody negative, C3 154 mg/dL, C4 46.1 mg/dL, rapid plasma reagin negative, and HIV negative. Those were all non-specific findings.
There was no evidence of systemic infection. The screening test for HIV and syphilis were negative. We could exclude acute rheumatic disease according to modified Jones criteria. All of the serologic markers for autoimmune disease were normal.
Three days after admission, we started steroid pulse therapy with prednisolone 60 mg/day and warfarin under the diagnosis of idiopathic aortitis with left renal vein thrombosis. After 3 days medication, his symptoms improved markedly. After 1 week, urinalysis showed the absence of proteinuria.
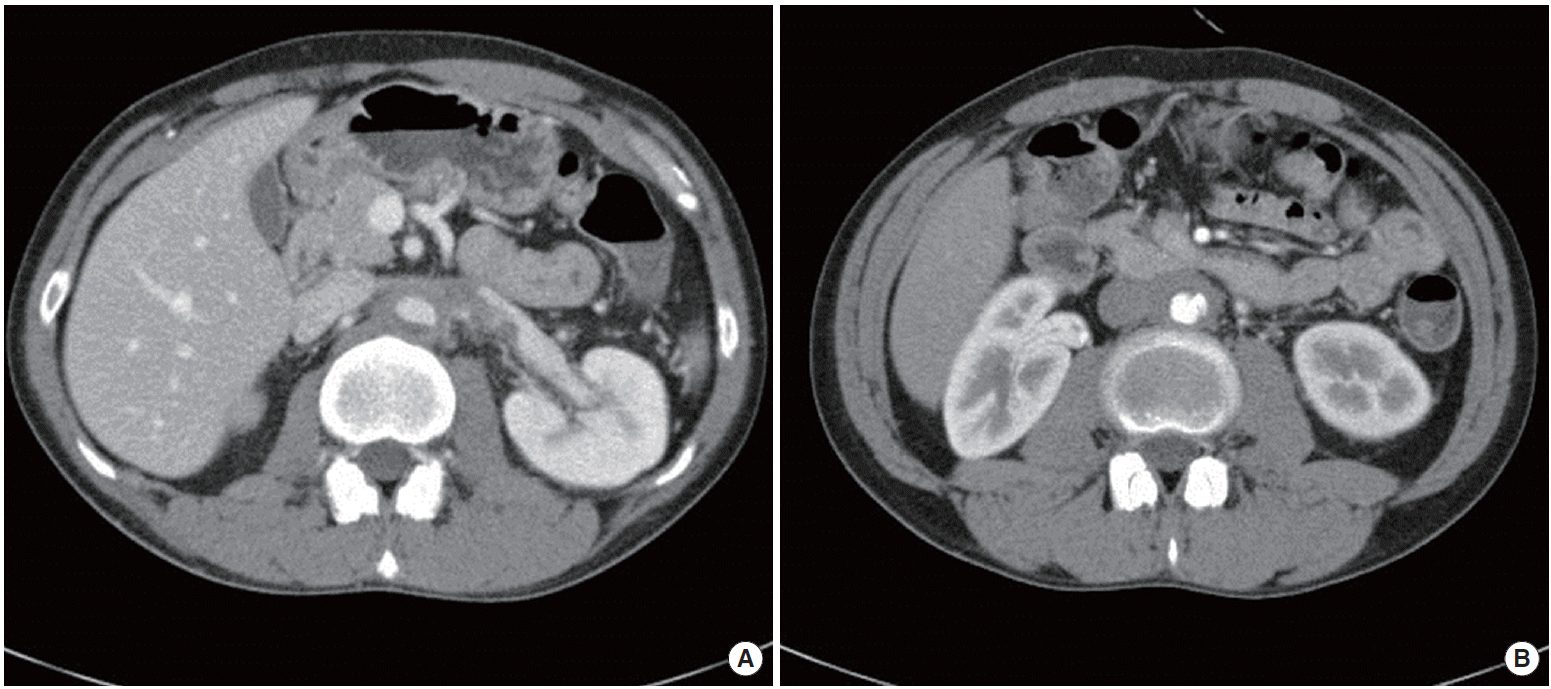
After 11 weeks of the treatment, we performed an abdomen CT with enhancement for follow-up. The CT demonstrated a decreased mural thickening of the abdominal aorta and resolved left renal vein thrombosis (Fig. 2). We prescribed prednisolone 60 mg/day for 12 weeks, and after that tapered it down by a half dose every 2 weeks. The patient will undergo abdominal CT at regular intervals in the future.
DISCUSSION
Aortitis is a pathologic term for the presence of inflammatory changes of the aortic wall, regardless of the underlying cause. Aortitis may be infectious or more commonly noninfectious [3]. In the present case, although a tissue biopsy of the aorta was not performed, we could exclude other types of aortitis including autoimmune aortitis, Takayasu’s arteritis, and GCA based on a serologic test and the history of the patient. Thus, it seems likely that the diagnosis in this case is “idiopathic aortitis”.
In large surgical cohort studies, the frequency of idiopathic aortitis made up 4.3% to 8.4% of all aortitis cases [4-6]. We could find one similar case to ours. The case was isolated idiopathic aortitis with left renal vein involvement consistent with renal infarction. In this case, a concentric periaortic soft tissue mass caused narrowing of the renal artery. Although they did not take a biopsy of the lesion, they could exclude other types of aortitis based on the serologic test and history. The patient was also treated with prednisolone 60 mg/day, resulting in gradual improvement signs and symptoms with progressive resolution of the periaortic rind in CT scan [7].
Female gender and active smoking are risk factors for development of idiopathic aortitis. There is no difference in the prevalence of hypertension, hyperlipidemia, or family history of any aortic aneurysm [8]. In this case, the patient was a social smoker. The clinical presentation of aortitis varies across a spectrum of symptoms and clinical signs, ranging from back or abdominal pain with fever to acute severe aortic insufficiency to an incidentally identified large aortic aneurysm [1,9].
Multi-detector CT with intravenous iodized contrast is usually the first image test requested due to its availability. It is highly sensitive and specific (84% and 100%, respectively). A mural thickening density mass of soft parts of the involved large artery is characteristic in CT findings. Magnetic resonance is recommended for patients who need repeated or follow-up examinations. Positron emission tomography is being used to diagnose aortitis. It is useful to follow the disease up and control response to treatment [10]. In our case, abdominal CT was performed and the scan showed homogenous arterial wall thickening in the form of a low density soft tissue mass surrounding the abdominal aorta.
The therapy for the aortitis was focused on the immediate treatment of aortic inflammation, infection, and reduction of its complications. In idiopathic aortitis, the optimal management is uncertain. Steroids are usually effective. It can induce remission of the clinical symptoms, normalization of the acute-phase reaction and reduction in size of the inflammatory mass. A number of immunosuppressive drugs, such as azathioprine, cyclophosphamide, and methotrexate, have been used as steroid-sparing agents, or in patients not responding to steroids alone or when steroids cannot be tapered [7]. The decision to treat with a course of glucocorticoid therapy should be considered on a case-by-case basis. Patients with isolated idiopathic aortitis require careful follow-up because small case series have identified a propensity toward aneurysm formation in other vascular beds over time [1]. In the reported case, steroid therapy was decided on because the thickened abdominal aorta wall compressed the left renal vein. A follow-up CT showed decreased aortic mural thickening, and there was no evidence of aneurysmal formation.
In conclusion, this case has the important clinical implication that idiopathic aortitis can compress renal vein and cause vascular thrombosis. We therefore present the rare case report of idiopathic aortitis of the abdominal aorta presenting as left renal vein thrombosis.