INTRODUCTION
Extramammary Paget’s disease (EMPD) is a rare cutaneous adenocarcinoma [1]. Most of patients with EMPD have a good prognosis, but invasive EMPD with regional metastasis has a poor prognosis [2]. A few cases show aggressive manifestations of EMPD with systemic metastasis [2]. Pulmonary tumor embolism (PTE) is a rare complication of malignant disease and difficult to diagnose ante mortem. On the other hand, in solid tumor, bone marrow metastasis is also a rare presentation. We present a successfully diagnosed with high suspicion and treated case of recurred EMPD in bone marrow alone with PTE, which presented with progressive dyspnea.
CASE REPORT
A 72-year-old male patient presented with anorexia, weight loss and progressive dyspnea of exertion over one month. Eight years earlier, he had been diagnosed of prostate cancer and he was treated with radical prostatectomy. Three years earlier, he was diagnosed for Paget’s disease in scrotum and received two steps operation because residual tumor was detected in resection margin. He was finally treated with wide excision and local flap. He was without evidence of disease on routine surveillance exam.
He had a 20 pack-year history of smoking but never had any breathing difficulty before. The physical examination including chest auscultation was unremarkable. He was afebrile with tachycardia of 108 beats/min, respiratory rate of 33/min, and blood pressure of 146/90 mm Hg. Arterial blood gas analysis showed paO2=59.4 mm Hg and paCO2=27.7 mm Hg while D-dimer levels were slightly elevated (5.87 μg/mL, reference range=less than 0.4 μg/mL). White blood cell, hemoglobin, and platelet count were 4,750/mm3, 13.5 g/dL, and 97,000/mm3, respectively. Prothrombin time was slightly prolonged to 15.4 seconds but partial thromboplastin time and fibrinogen were in normal range.
Pulmonary function test showed normal ventilator pattern, but decreased diffusing capacity of the lungs for carbon monoxide (DLCO=68%). Contrast enhancement computed tomography (CT) scan did not show any intraluminal filling defects of pulmonary arteries and deep veins of low extremities (Fig. 1). Severe pulmonary hypertension with relatively preserved right ventricular function was documented with echocardiography; estimated pulmonary artery systolic pressure (ePASP) was 80 mm Hg. Despite the negative CT scan result, pulmonary thromboembolism was suspected. Ventilation-perfusion lung scans demonstrated multiple perfusion defects with normal ventilation (Forte; ADAC-Philips, Holt, MO, USA) (Fig. 2). These findings were consistent with microscopic pulmonary thromboembolism.
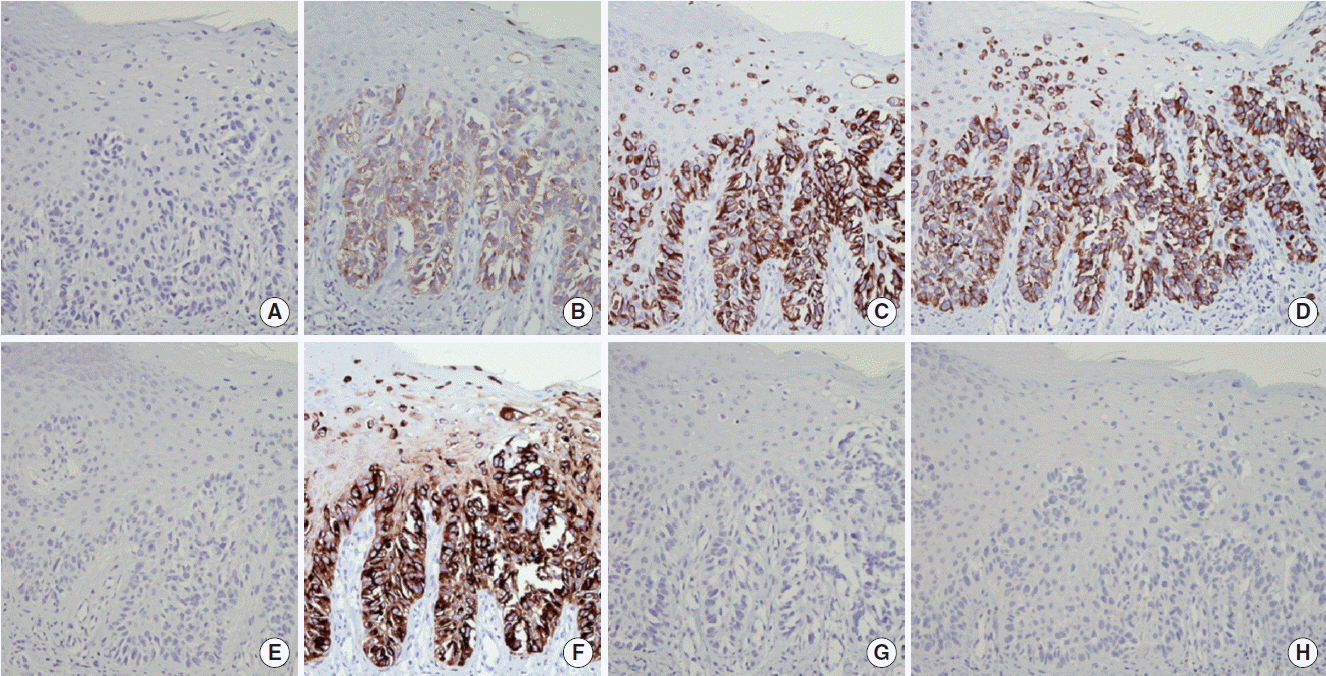
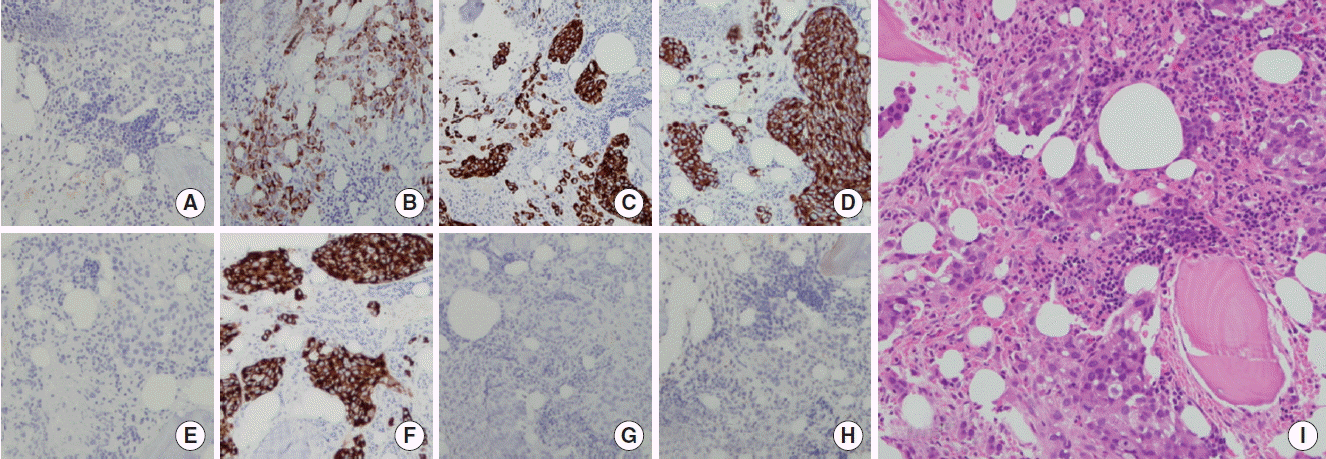
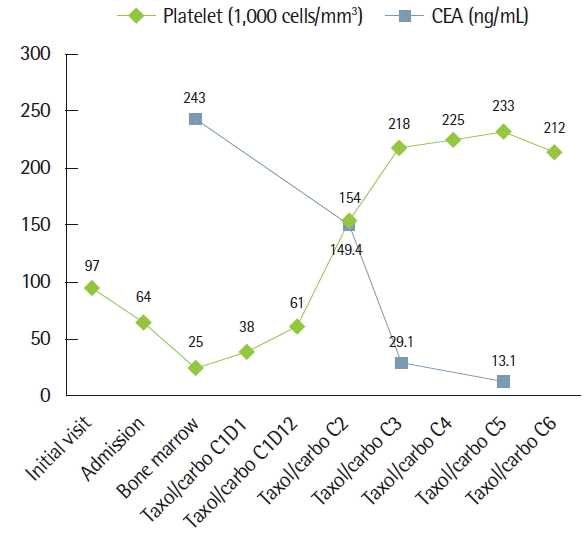
Anticoagulation therapy was suggested, but was postponed because progressive thrombocytopenia was detected. Multiple aggregations of large sized abnormal cells were observed in bone marrow specimen, suspecting malignancy. These tumor cells were same histology and immunohistochemical staining pattern (caudal-related homeobox 2 [CDX2, −], carcinoembryonic antigen [CEA, +], cytokeratin [CK] 7 [+], CK19 [+], CK20 [−], epithelial membrane antigen [EMA, +], prostate-specific antigen [PSA, −], thyroid transcription factor-1 [TTF-1, −]) with previous EMPD of scrotum (Figs. 3, 4). There was no lesion that suggests distant metastasis in additional whole body positron emission tomography-computed tomography-CT scan. Serum CEA was elevated to 243 ng/mL (reference range, 0 to 5 ng/mL) and serum prostate-specific antigen level was normal. Therefore, a diagnosis of recurred EMPD in bone marrow alone with microscopic PTE was made.
The patient received paclitaxel/carboplatin chemotherapy with respiratory support in intensive care unit. Four days after chemotherapy, the oxygen demand decreased and the patient was transferred to general ward. The platelet count recovered after 2 weeks (Fig. 5). After 6 cycles of chemotherapy, ePASP decreased to 34 mm Hg and sequential bone marrow biopsy showed decreased tumor cell infiltration. Unfortunately, the patient refused more paclitaxel/carboplatin chemotherapy cycle due to financial difficulties. One month after he decided to receive alternative 5-fluorouracil/cisplatin chemotherapy. After 3 cycles of 5-fluorouracil/cisplatin chemotherapy, new hepatic metastasis was detected and confirmed by liver biopsy. We tried docetaxel chemotherapy to treat his progressive disease but it did not respond. Finally, he died of hepatic failure from Paget’s disease hepatic involvement 10 months after recurrence.
DISCUSSION
EMPD is a rare cutaneous malignancy from intraepidermal region around apocrine gland. Distal metastasis beyond regional lymph nodes is rare and only few cases were reported [2,3]. EMPD within the epidermis may progress to dermally invasive adenocarcinoma. Then this might metastasize to locoregional lymph nodes and distant sites [4]. The depth of invasion was most significant prognostic factor [2]. Few cases were reported as bone marrow involvements. Most of them presented with locoregional lymph node involvement. One case of minimally invasive Paget’s disease of the vulva, which presented by local recurrences and bone marrow metastases 6 years after excision, was reported [5]. Recurrence of EMPD of scrotum in liver and bone marrow 3 years after initial treatment was reported [6]. In our patient’s case, minimal dermal invasion was detected but lymph node dissection was not done. At the time of recurrence, no evidence of local or nodal recurrence was detected. It is possible that some tumor cells could be introduced into lymphatics during surgery and some of them were able to survive in the vascular system [5]. After long incubation period, sequential hematogenous spread of tumor cells from bone marrow to pulmonary vasculatures might have caused respiratory distress.
Kane et al. [7] described four types of tumor involvement of the pulmonary vessels: (1) tumor emboli of the large pulmonary arteries and branches, (2) lymphatic dissemination, (3) microscopic tumor emboli in the small arteries or arterioles (microscopic PTE), and (4) combinations of above. Diagnosis of PTE requires a high index of suspicion. Rapid deterioration of patient makes confirmative diagnosis difficult. There are few cases reports in the literature where the diagnosis of PTE was made ante mortem [8]. If clinical diagnosis of microscopic PTE is made and severity of respiratory distress is rapidly progressing, immediate treatment is more important than confirmative diagnosis. In our case, suspicious PTE was diagnosed using all available image modalities including echocardiography, chest CT scan, and ventilation-perfusion lung scans, although there was not biopsy-confirmed. Improving dyspnea and O2 demand after appropriate chemotherapy could confirm the proper diagnosis of PTE.
Standard chemotherapy regimens for locally advanced or metastatic EMPD have not been established [6]. There is no clinical trial for the efficacy of chemotherapy in locally advanced or metastatic EMPD due to scarce incidence. Only case reports with several agents were reported including 5-fluorouracil, platinum-base agents, mitomycin-C, docetaxel, and vincristine [6,9–12]. Clinical responses from these case reports were various. In our case, paclitaxel and carboplatin was applied in first line chemotherapy. Response to the chemotherapy was very dramatic. After progression on first-line chemotherapy, 5-fluorouracil/cisplatin and docetaxel was applied. Finally, he died of progressive liver involvement of disease in 10 months after recurrence, which is a little long survival period.
This case is a rare presentation of bone marrow metastasis with PTE secondary to EMPD of scrotum, which was properly diagnosed with high suspicion and was successfully treated with immediate chemotherapy.