INTRODUCTION
Clostridium difficile infection (CDI) is a healthcare associated infection of growing importance that occurs after prolonged hospitalization and exposure to broad-spectrum antibiotics [1,2]. CDI has a wide range of clinical manifestations, including asymptomatic infection, mild diarrhea, fulminant pseudomembranous colitis (PMC), and even death [3,4]. The incidence and severity of CDI in Korea, is increased in recent years [5]. According to the national wide study, the total incidence of CDI in Korea was 1.7 cases/1,000 adult admissions in 2004, and 2.7/1,000 adult admissions in 2008 (P=0.028) [6]. Most clinical practice guidelines recommend oral metronidazole as the initial therapy for the treatment of mild to moderate CDI, then oral vancomycin reserved for severe CDI, or intolerance to or failure to respond to metronidazole treatment [7,8]. However, in a real world clinical setting with severe CDI, the question to whom to use oral vancomycin is still a challenging one, due to the risk of emergence of the vancomycin-resistant enterococci and the high cost of vancomycin [9,10]. There is an issue about the over-enrollment of severe CDI with current criteria which suggests potential efficacy of less active agents for these “severe” patients [11] (Table 1): for example, a patient aged over 60 years old presenting with a fever (body temperature >38.3°C) will be classified as having severe CDI regardless of any co-morbidity. In actual clinical practice, oral metronidazole is prescribed to many patients who would be formally classified as presenting with severe CDI due to its tolerability and low cost. Therefore, we compared the clinical efficacy (clinical cure, recurrence, and 30-day mortality) of oral metronidazole with that of oral vancomycin in patients with severe CDI, and evaluated the risk factors associated with treatment failure.
MATERIALS AND METHODS
1. Study design and patients
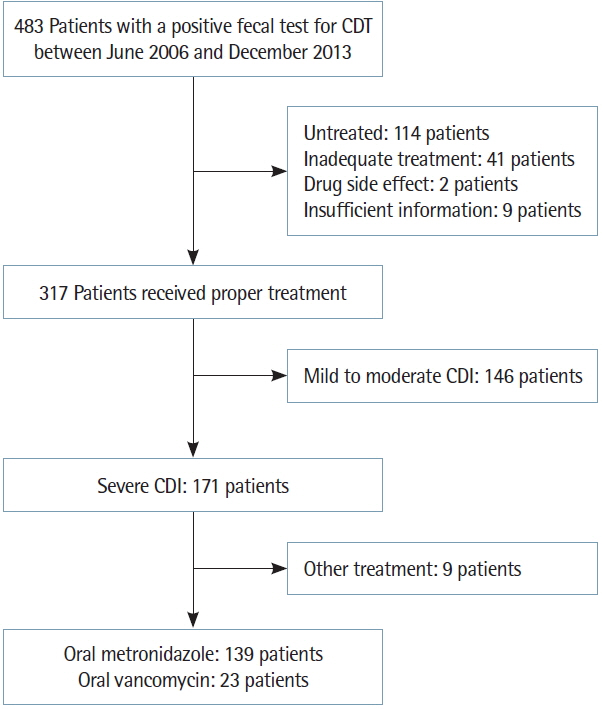
This retrospective study was performed at a 749-bed teaching hospital between June 1, 2006 and December 31, 2013. No outpatients were included in this study. Patients with mild to moderate CDI, no treatment, inadequate treatment (other antibiotics, insufficient dose, and insufficient duration) and insufficient information were excluded. The diagnosis of CDI in patients with diarrhea was confirmed by a positive enzyme-linked immunosorbent assay (ELISA) for C. difficile toxins A/B (TOX A/B QUIK CHEK; TECHLAB, Blacksburg, VA, USA). Four hundred and eighty-three patients were diagnosed with CDI. Among these patients, a total of 171 patients were included in the study, while the other 312 patients were excluded in accordance with above criteria. Among these 171 patients with severe CDI, nine patients were excluded because they received other treatments (e.g., intravenous metronidazole, oral vancomycin together with intravenous metronidazole, or vancomycin enema) (Fig. 1). We collected baseline characteristics from the patients’ medical records, such as age; sex; combined comorbidity; intensive care unit (ICU) admission; the use of antibiotics, immunosuppressive agents, histamine-2 (H2) antagonist, proton pump inhibitor or chemotherapy agents; laboratory findings including white blood cell (WBC) count, serum albumin level, C-reactive protein level; and imaging findings including sigmoidoscope and abdominal X-ray in the case of examinations performed. Patients received either oral metronidazole (500 mg 3 times per day) or oral vancomycin (either 125 mg 4 times per day or 250 mg 3 times per day) for at least 10 days.
The Institutional Review Board of the Soonchunhyang University Hospital approved this study and waived the requirement for informed consent (IRB approval no., 2014-05-010).
2. Definition
The severity of CDI was assessed using the CDI severity score system from resource of the American College of Physicians [11] (Table 1). The classification of severity was based on patient data from 2 days before diagnosis until 2 days after diagnosis. The primary outcomes were clinical cure, recurrence, and 30-day mortality. Clinical cure was defined as the resolution of diarrhea by day 6 of treatment and no further requirement for therapy for CDI from the second day after the end of the therapy. Treatment failure was defined as persistence of diarrhea and need for different or additional therapy for CDI, based on the opinion of clinicians. Recurrence was defined as diarrhea recurrence with a positive ELISA result for C. difficile toxin A/B, within 2 months of initial clinical cure [12]. The 30-day mortality was defined as death from any cause within 30 days of a diagnosis of CDI.
3. Statistical analysis
All statistical analyses were performed using SAS ver. 9.3 (SAS Institute Inc., Cary, NC, USA). Patients were divided into an oral metronidazole group and an oral vancomycin group. Comparison of the groups was performed using the independent Student t-test or the Wilcoxon rank sum test for continuous variables, and the chi-square test or Fisher’s exact test for categorical variables. To estimate independent risk factors for treatment failure, logistic regression analysis was used. All clinical and laboratory parameters were a prior tested for univariate analysis. The parameters with a P-value of less than 0.1 and those with potentially biologic meanings were considered in a multivariate analysis. A P-value of <0.05 was considered to be statistically significant.
RESULTS
1. Patient characteristics and clinical outcomes
A total of 162 patients with severe CDI were studied: 139 patients received oral metronidazole, and 23 patients received oral vancomycin. Table 2 reports the characteristics of the study populations. The mean age of the patients was 70.0±11.5 years. Eighty-six patients were male. There was no significant difference between oral metronidazole group and oral vancomycin group in terms of age adjusted Charlson comorbidity index (oral metronidazole, 5.04; oral vancomycin, 5.96; P=0.053). Oral vancomycin group showed more proportion of diagnosis of end stage renal disease and higher WBC level. There were no patients with vancomycin-resistant enterococci in the oral vancomycin group. A total of 46 patients (oral metronidazole, 34 patients; oral vancomycin, 12 patients) underwent sigmoidoscope. The result of examination was PMC in 41 patients (oral metronidazole, 29 patients; oral vancomycin, 12 patients).
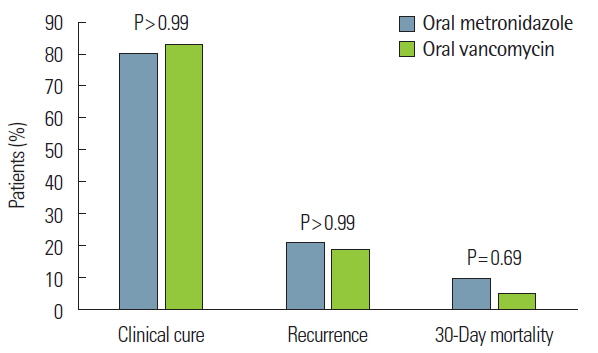
The overall clinical outcomes are shown in Fig. 2. The clinical cure rate was 79.86% (111 of 139 patients) in patients receiving oral metronidazole and 82.61% (19 of 23 patients) in those receiving oral vancomycin (P>0.99). Following treatment failure of oral metronidazole, clinical cure was achieved by changing to treatment with oral vancomycin (n=23, 82.14%), intravenous metronidazole (n=3, 10.71%), or both oral vancomycin and intravenous metronidazole (n=2, 7.14%). The recurrence of CDI occurred in 27 of 139 patients (20.77%) treated with metronidazole and in four of 23 patients (18.18%) treated with oral vancomycin (P>0.99). There was no significant difference between oral metronidazole and oral vancomycin treatment in the rate of 30-day mortality (9.35% [13 of 139 patients] vs. 4.35% [one of 23 patients], P=0.69). Only one case with oral metronidazole was CDI-related death. The patient expired due to CDI with neutropenia during hospitalization for gastric cancer treatment.
2. Factors associated with treatment failure in severe Clostridium difficile infection patients
We analyzed the characteristics of severe CDI patients with respect to whether they had treatment failure. Age; gender; diagnosis of diabetes mellitus, hypertension, or end stage renal disease; ICU admission; duration of ICU stay; use of any antibiotics; antibiotic maintenance during treatment; use of an immunosuppressive agent; use of a proton pump inhibitor; use of a chemotherapy agent; operation; serum albumin level; WBC level; finding of PMC on sigmoidoscope; and finding of ileus on simple abdominal X-ray were not related to treatment failure. In addition, the type of medication for treatment of CDI was not related to treatment failure. We analyzed the variables that showed trend towards being related to treatment failure (P<0.1) with multivariate analysis. The use of H2 antagonist (P=0.0032; odds ratio [OR], 4.34; 95% confidence interval [CI], 1.64–11.51) and the presence of a fever (body temperature >38.3°C: P=0.049; OR, 2.4; 95% CI, 1.0–5.86) were found to be significantly correlated with treatment failure (Table 3).
DISCUSSION
The Society for Healthcare Epidemiology of America/Infectious Diseases Society of America guideline and the American Gastroenterology Association recommend oral vancomycin as the first-line agent for the treatment of severe CDI patients [7,13].
Oral metronidazole treatment is generally considered to be less effective than oral vancomycin treatment, because cases of elderly patients with severe comorbidities are increasing, and because the use of broad-spectrum antibiotics (new fluoroquinolones and cephalosporins) is also increasing [14–16]. Metronidazole resistance, the emergence of the BI/NAP1/027 (ribotype 027) strain of C. difficile, and poor concentration of oral metronidazole in colon are also potential causes of the decreasing efficacy of oral metronidazole.
The emergence of the hypervirulent BI/NAP1/027 (ribotype 027) strain of C. difficile is associated with more severe disease due to increased toxin production. In 2009, Tae et al. [17] reported the first case of C. difficile polymerase chain reaction (PCR) ribotype 027 infection refractory to oral metronidazole therapy in Korea. However, the proportion of this hypervirulent strain is low [18]. Kim et al. [19] performed a prospective study that found that binary toxin-producing C. difficile infection, including ribotype 027, was not common in Korea and that these isolates were highly susceptible to metronidazole and vancomycin.
Metronidazole-resistant strains of C. difficile are rare. In 1999, for the first time, Wong et al. [20] detected a metronidazole-resistant strain of C. difficile in a patient with CDI. In Korea, Kim et al. [21] reported that no metronidazole resistance was detected. Metronidazole resistance does not appear to be increasing [22]. Therefore, the emergence of the BI/NAP1/027 strain and metronidazole resistance does not have a critical influence on the efficacy of oral metronidazole treatment in severe CDI patients in Korea.
The antibiotic concentration in the colon is very important for the treatment for CDI. The difference in treatment effect for CDI between oral metronidazole and oral vancomycin is due to the difference in the absorption rate in the colon. When oral metronidazole is administered, it is absorbed rapidly and almost completely, with only 6%–15% of the drug metabolites excreted in the stool. This concentration rate of metronidazole in stool reflects the rate of its secretion in the colon. Once treatment of CDI is initiated, the stool concentration rate of metronidazole decreases rapidly from 9.3 μg/g in watery stools to 1.2 μg/g in formed stools [23]. In asymptomatic C. difficile carriers, metronidazole is not detected in stool [24]. In contrast, oral vancomycin is poorly absorbed; thus, fecal concentrations are maintained throughout treatment. Johnson et al. [24] reported that the fecal concentration rate after oral vancomycin administration (125 mg administered 4 times daily) is generally >1,000 μg/g. Oral metronidazole treatment is reported to fail, because the concentration of metronidazole in colon was slowly increased but was not maintained at a consistent rate in colon [10,22,25]. If a patient shows no clinical response to oral metronidazole, clinicians should consider changing medications (e.g., oral vancomycin or intravenous metronidazole). In our study, all treatment failure patients receiving oral metronidazole achieved a clinical cure after switching to oral vancomycin or intravenous metronidazole.
Especially, patients with severe complicated CDI, with symptoms including hypotension, shock, and/or megacolon, do not have enough time to wait until metronidazole treatment will take effect and a clinical cure will be achieved [7]. Thus, severe complicated CDI patients should be treated in accordance with current clinical practice guidelines, based on high dose vancomycin (500 mg 4 times daily) [7].
In our study, H2 antagonist treatment and fever (body temperature >38.3°C) were related to treatment failure. There are some reports supporting the association between H2 antagonist therapy and CDI treatment failure. The mechanism of how H2 antagonist exposure contributes to an increased risk of CDI treatment failure is not yet clear. However, it is strongly suspected that the effect of H2 antagonist in suppressing gastric acid secretion aids the survival of C. difficile [26–28]. Dial et al. [29] reported that the use of acid-suppressive therapy is associated with an increased risk of CDI. However, Jung et al. [30] did not find an association between the use of H2 antagonist and metronidazole treatment failure. Further studies on the role of acid-suppressive therapy in CDI treatment appear to be necessary.
This study had some limitations: it was retrospective, taking place in a single tertiary medical center. The number of patients was relatively small. In addition, the hospital used a toxin assay for diagnosis, which is less sensitive than diagnosis by C. difficile PCR and C. difficile culture.
In conclusion, we found that in treating severe CDI patients, oral metronidazole was an effective treatment in addition to oral vancomycin. Therefore, oral metronidazole can be viewed as a valid clinical option for treating not only patients with mild to moderate CDI but also those with severe CDI.