A Case of Extremely Premature Baby with Persistent Metabolic Acidosis, Hypoglycemia and Dyselectrolytemia Induced by Liposomal Amphotericin B
Article information
Abstract
Amphotericin B is widely used in the treatment of neonatal invasive fungal disease. Liposomal amphotericin B (LAmB) is now preferred over conventional amphotericin B deoxycholate due to its lower kidney toxicity. However, it is essential to monitor the possible side effects of LAmB when treating extremely premature infants. Herein, we present a case of an extremely premature infant who exhibited persistent metabolic acidosis, hypoglycemia, and dyselectrolytemia after administration of LAmB for a suspected fungal infection.
INTRODUCTION
Neonatal sepsis remains a major cause of neonatal mortality and morbidity in preterm infants. Therefore, recognition of early symptoms and the initiation of appropriate treatment are essential for pediatricians working in the neonatal intensive care unit (NICU) [1]. Those born at the lowest gestational ages are known to be more vulnerable to suffer from sepsis among very preterm infants [2]. Not only specific bacteria but also pathogenic fungi, such as Candida spp. can cause systemic infection. Approximately 15% of extremely low birth weight neonates are affected by systemic fungal infection during NICU care [3]. European Society for Clinical Microbiology and Infectious Diseases and Infectious Diseases Society of America published guidelines for the candidiasis treatment in 2012 and 2016, respectively [4,5]. They both recommend either fluconazole or amphotericin B (amphotericin B deoxycholate [DAmB] or liposomal amphotericin B [LAmB]) for neonatal invasive candidiasis treatment.
Amphotericin B acts by binding to ergosterol in the fungal membrane, a process that results in cell death. Since renal tubular cells are high in cholesterol, nephrotoxicity with electrolyte imbalance is the major side effect of amphotericin B [6]. DAmB, the conventional amphotericin B, was approved for use in both children and adults. However due to its nephrotoxicity, lipid formulation of amphotericin B (LAmB or AmBisome) are increasingly used [7]. Even though LAmB is known to be well tolerated even in premature infants with mild and transient nephrotoxicity, its clinical safety and pharmacokinetics are still not fully understood.
Herein, we report a case of an extremely premature infant who showed persistent metabolic acidosis, hypoglycemia and dyselectrolytemia after using LAmB for suspected fungal infection.
CASE REPORT
A female newborn was born by cesarean section at 27+1 weeks’ gestation to a 26-year-old mother. The mother gave birth due to severe oligohydramnios, with no maternal history of smoking, alcohol, or teratogenic exposure. The baby had severe intrauterine growth retardation, and her birth weight, birth length and head circumference were 360 g (<3rd percentile), 26.0 cm (<3rd percentile), and 20.5 cm (<3rd percentile), respectively. At birth, her Apgar scores were 5 and 7 at 1 minute and 5 minutes, respectively. A physical exam revealed no other abnormalities. Since she was born as extreme low birth weight infant, she was admitted to our NICU for further evaluation and treatment.
The infant was immediately intubated with surfactant and received support through high-frequency oscillatory ventilation (HFOV). On the 29th day of life (DOL), she remained clinically stable on HFOV with successful breast milk feeding of 4 mL every 3 hours. However, a significant clinical deterioration, marked by hypotension, repeated bradycardia, and desaturation, was observed on the 29th DOL when her weight was 590 g. A blood test revealed a C-reactive protein level of 36.97 mg/L (normal range, 0–5 mg/L) and a metabolic acidosis with a pH of 7.11, partial pressure of carbon dioxide (pCO2) 49.7 mm Hg, partial pressure of oxygen (pO2) 112.2 mm Hg, and a base excess of −13.3 mEq/L. Given the rapid aggravation of her clinical symptoms, we suspected a systemic infection. We started LAmB (5 mg/kg/day) combined with vancomycin, meropenem, and inotropics (dopamine, dobutamine, epinephrine).
On 33rd DOL, her vital signs became stable and no specific pathogen was found from 29th DOL blood culture test. However, metabolic acidosis (anion gap <15 mEq/L) persisted despite continuous infusion of sodium bicarbonate (3–5 mEq/kg/day). Suspecting of nephrotoxicity induced by LAmB, we stopped the drug 96 hours after first dose (33rd DOL) (Fig. 1). After stopping LAmB, glucose infusion rate to maintain euglycemia has increased to peak 15–18 mg/kg/min until 36th DOL (Fig. 2). Urine analysis performed on 36th DOL revealed pH 5.0, urine glucose 4+ (2,000 mg/dL), and positive urine anion gap. Platelet count fell to 23,000/μL on 36th DOL. Metabolic acidosis persisted that more than 10 mEq/kg/day of sodium bicarbonate was needed to maintain a normal pH (Fig. 2). Aggravated hyponatremia and hypocalcemia were noticed on 40th DOL, and 7–10 mEq/kg/day of sodium and 4–4.5 mEq/kg/day of calcium were needed to correct electrolyte imbalance (Fig. 2). Blood urea nitrogen and creatinine level were always normal with normal urine output during the hospital days. Intravenous sodium bicarbonate was gradually reduced and finally discontinued on 57th DOL. Hypoglycemia as well as dyselectrolytemia reached to normal level simultaneously with correction of serum metabolic acidosis (Figs. 1, 2).
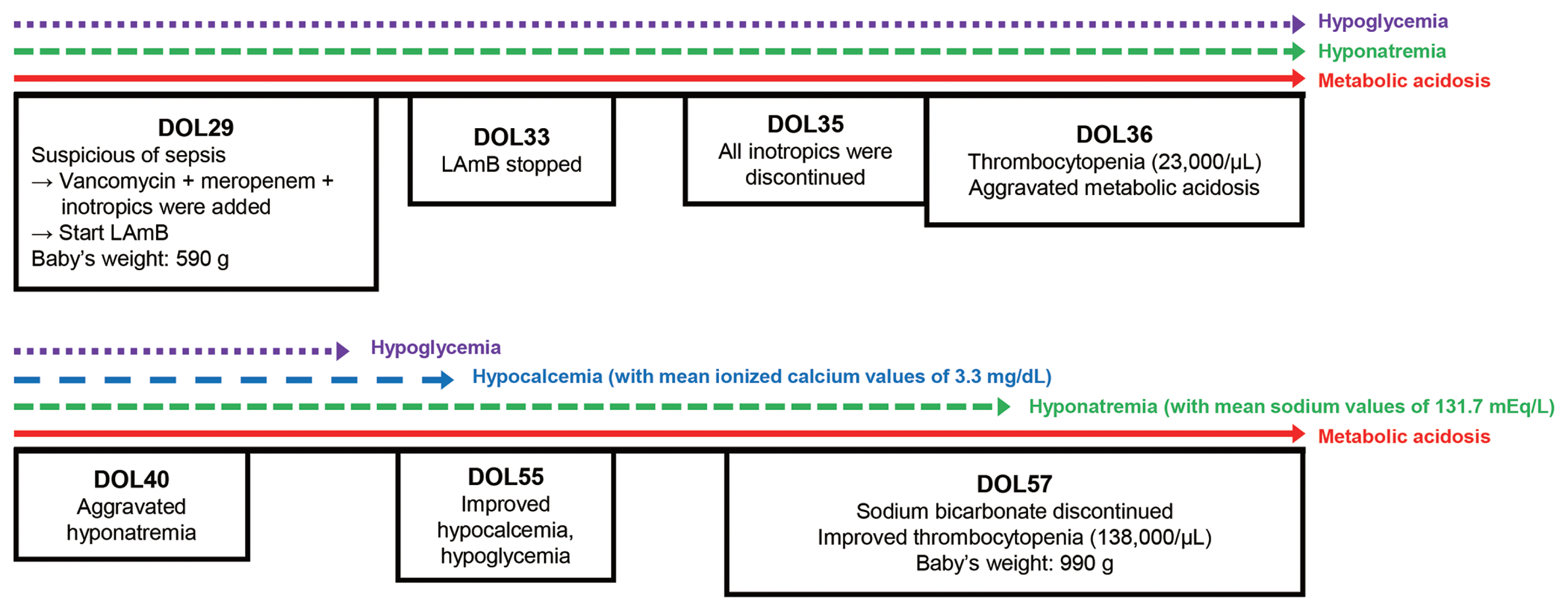
Clinical course of patient after initiating liposomal amphotericin B (LAmB) treatment. DOL, day of life.
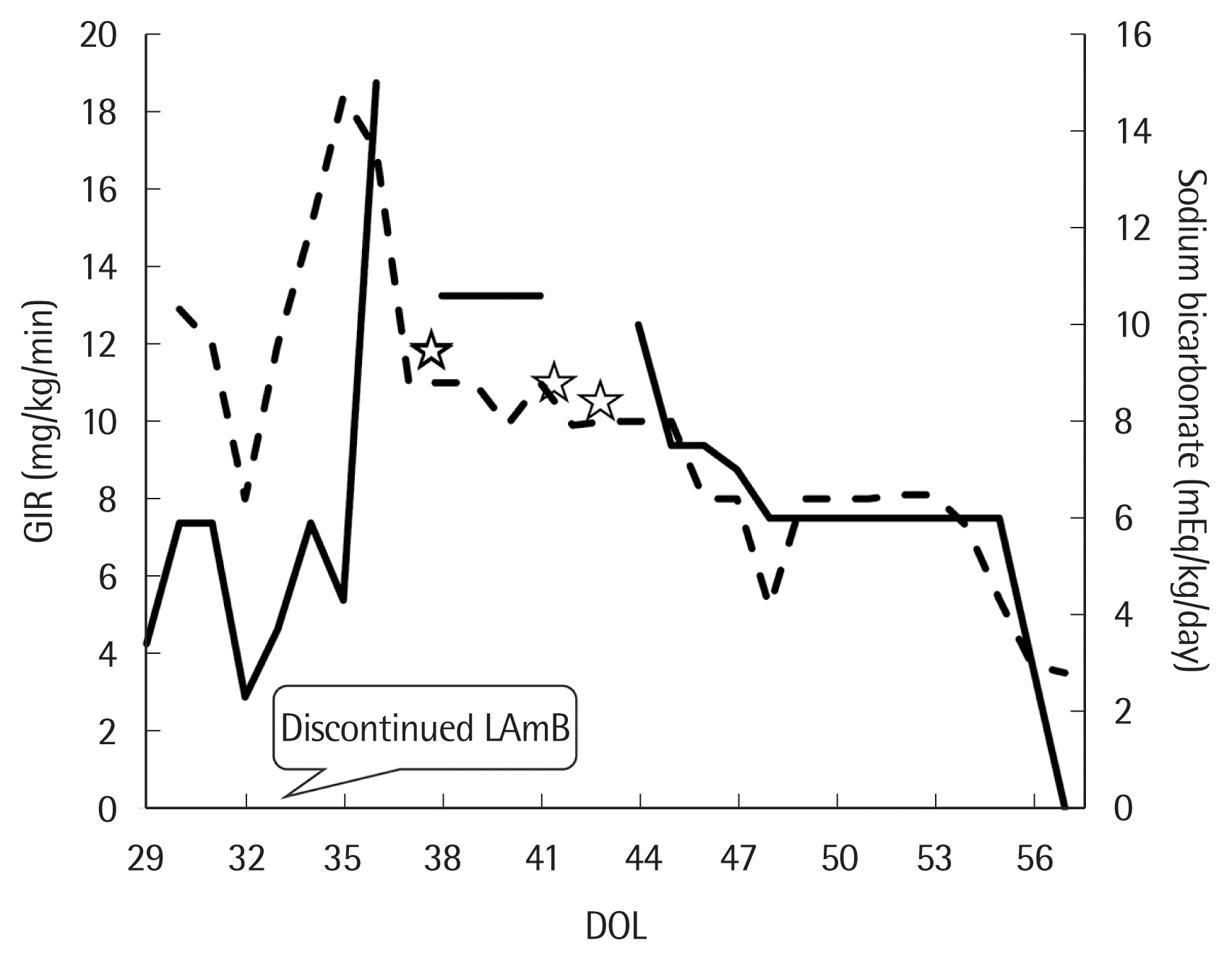
The solid line indicates the amount of glucose infusion rate (GIR) needed to maintain euglycemia. Dotted line represents the total amount of sodium bicarbonate administered to control metabolic acidosis. On 37th, 42th, and 43th day of life (DOL) (stars), we tried to stop sodium bicarbonate but metabolic acidosis persisted until 57th DOL. LAmB, liposomal amphotericin B.
The patient in now 10 months old and has been transferred to another hospital due to severe pulmonary hypertension. Metabolic acidosis, hypoglycemia, and dyselectrolytemia did not recur after 57th DOL.
This study adhered to the tenets of the Helsinki Declaration ( http://www.wma.net/en/30publications/10policies/b3/) and was approved by the Research Ethics Committee of the Institutional Review Board (IRB) of our institution (approval no., IRB 2023–08–043).
DISCUSSION
Mortality rates in the NICU for invasive candidiasis are reported up to 50% in very low-birth infants. Neurodevelopmental impairments can occur, particularly following Candida meningitis [8,9]. Therefore, early initiation of treatment when diagnosing or even suspecting a systemic fungal infection before getting culture results is imperative. Although we have two recent guidelines regarding neonatal candidiasis treatment [4,5], they are limited by their reliance on small prospective or retrospective database studies. Currently, off-label antifungal use is quite common in the NICU, and the selection of antifungal drugs usually depends on the neonatologist’s decision. As previously mentioned, amphotericin B itself is a polyene characterized by lipophilic and hydrophilic regions that can bind to the cholesterol in the renal tubular cells, resulting in renal damage [7]. It usually affects distal tubular epithelium causing renal tubular acidosis (RTA) with electrolyte imbalance such as hypokalemia. Nephrotoxicity depends on cumulative dose which can lead to renal failure [9].
LAmB, one of the lipid formulations of amphotericin B, was invented to ameliorate the renal toxicity of conventional amphotericin B. It is now Food and Drug Administration-approved for use in children older than 1 month of age [10]. Despite the absence of pharmakokinetics data for neonates and no information on optimal dosing regimens, LAmB is increasingly used in the NICU for premature infants with suspected or proven invasive fungal infection. Juster-Reicher et al. [11] reported that a high dose (5–7 mg/kg/day) of LAmB was effective in 95% of 41 episodes of systemic candidiasis occurring in 37 neonates (28 were extremely low birth infants). It showed that fungal eradication was more rapid in patients treated early with high doses of LAmB as first-line therapy. In another study, renal function impairment was analyzed among 71 very low-birth infants with systemic fungal infection. A recommended dose of 3–5 mg/kg/day of LAmB was used. The mean duration of treatment was 14±9 days, with a mean cumulative dose of 58±25 mg/kg. Renal compromise, defined as hypokalemia and/or elevated serum creatinine levels and/or decreased urine output, occurred in only two out of 71 (2.8%) treated patients [7]. However, there are still concerns regarding the use of LAmB in extremely premature babies, especially those with intrauterine growth retardation. This is because there is no large safety data available in this physiologically and developmentally immature group. A recently published case report, used 5 mg/kg of LAmB in a neonate born at 25 weeks of gestation with a birth weight of 680 g to eradicate Candida albicans grown from cerebrospinal fluid culture. The baby then showed hyponatremia, hypokalemia, and hypomagnesemia, and these adverse effects were resolved with drug withdrawal [12]. In our case, we suspected RTA as the cause of prolonged metabolic acidosis, which required a high dose of sodium bicarbonate due to the use of LAmB. The serum potassium level remained within the normal range, but hyponatremia, hypoglycemia, and hypocalcemia persisted. Identifying the exact type of RTA was challenging in this case. Proximal renal tubular acidosis (pRTA) is characterized by bicarbonate wasting, whereas distal renal tubular acidosis (dRTA) results from an acidification defect [13]. Amphotericin B-induced tubulopathy typically presents as dRTA with severe hypokalemia and a high urine pH (>6.5). However, our patient exhibited a normal potassium level while requiring a high dose of alkali, consistent with pRTA. We concluded that possibly both types of RTA might have occurred due to the immaturity of the patient’s kidneys. It was a limitation that urine analysis was challenging due to the small size of the baby. Additionally, LAmB was administrated before obtaining blood culture results, and the result was normal, likely due to a small blood sample.
Early initiation of antifungal agents in cases of suspected or diagnosed fungal infection is crucial to prevent fatal outcomes. Although LAmB typically induces mild and transient adverse effects in neonates, it can pose a potential fatality risk to extremely premature infants. Therefore, close monitoring, including periodic blood tests, while maintaining adequate hydration and sodium intake is imperative when using LAmB in this vulnerable population. Larger prospective studies examining the safety of LAmB use in extremely premature infants will be necessary.
Notes
No potential conflict of interest relevant to this article was reported.