Idiopathic Neuralgic Amyotrophy in a Child Treated with Integrated Rehabilitation Program: A Case Report
Article information
Abstract
Idiopathic neuralgic amyotrophy (INA) in children is a rare peripheral neuropathy showing distinctive clinical features. An 8-year-old boy presented with left shoulder pain and weakness lasting 4 weeks. He had learned Taekwondo and swimming before the onset of pain. A fever had developed 10 weeks before onset but had completely resolved after medication. Initially, he was diagnosed with shoulder subluxation and underwent successful reduction. However, he continued to complain of persistent pain and weakness. All the studies indicated normal findings, except for the electrodiagnostic assessment, which revealed partial brachial plexopathy. A rehabilitation program was performed. It consisted of physical and occupational therapy twice a week. After 5-month rehabilitation, the patient remarkably improved in both neurologic and functional status. INA should be considered and evaluated in cases with abrupt onset of shoulder pain and weakness in children. Also, an integrated rehabilitation program during the subacute phase can be helpful to facilitate neurologic and functional recovery and reduce sequelae.
INTRODUCTION
Neuralgic amyotrophy (NA) is a peripheral neupathy demonstrating a characteristic clinical course. Classic NA manifests as sudden severe pain around the shoulder. As initial pain subsides and/or is replaced by less severe neuropathic or musculoskeletal-type pain, flaccid paralysis and subsequent atrophy appear in the shoulder girdle muscles [1].
The incidence of classic NA has been reported to be 1–3 per 100,000 to 1 per 1,000. The reason for such differences in incidence may be due to the unfamiliarity of NA and clinical similarity of NA to more common diseases. Furthermore, NA in children shows lower rates of initial shoulder girdle area pain as compared with the adult. Therefore, early awareness of the disorder in children is difficult, leading to frequent misdiagnosis or longer time to diagnose [2–4].
Various tests are needed to differentiate NA from other neuromuscular diseases. Among them, electrodiagnostic assessment is valuable for confirmation of the diagnosis, and estimation of severity and prognosis of the neuropathy [1,5].
Although there is no evidence-based treatment protocol, several management strategies are suggested. Some studies report that an immunotherapy could be effective for pain control and neurologic recovery. Additionally, a multidisciplinary rehabilitation program is suggested for functional improvement and reduction of musculoskeletal complications [6–8].
We present a case of idiopathic neuralgic amyotrophy (INA) in a child demonstrating the clinical improvement after an integrated rehabilitation program within 6 months of the onset.
CASE REPORT
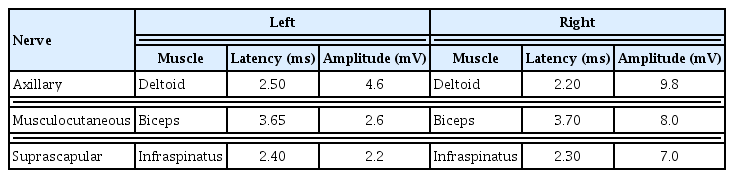
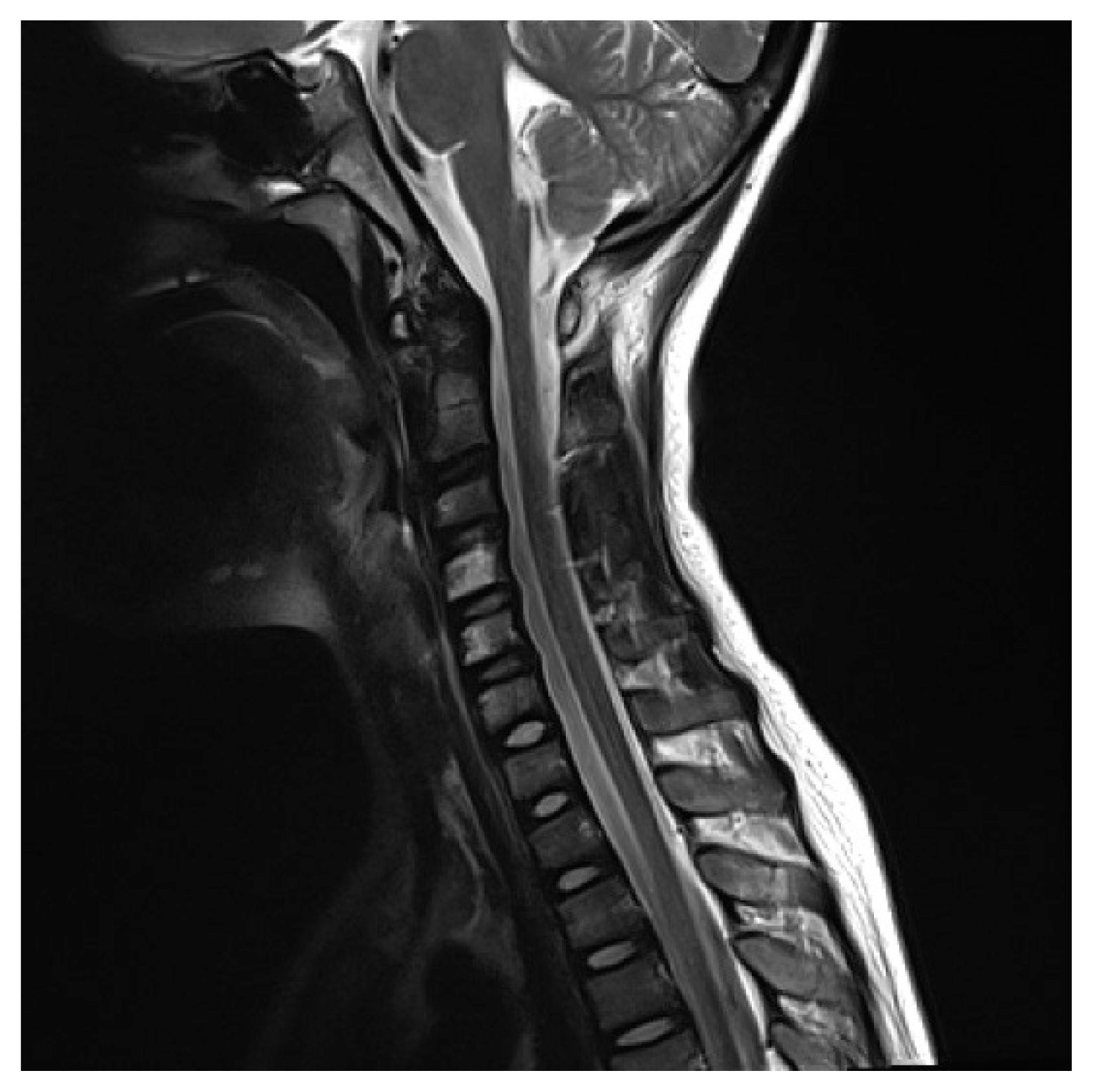
An 8-year-old boy visited the emergency room because of pain in the left shoulder girdle for 4 weeks. Two weeks after initial pain, pain had improved slightly but he could not raise his shoulder. There was no family history or past history. His weekly routine included Taekwondo lessons for last 3 years, and swimming for 4 weeks before the onset of pain. He had a fever 10 weeks before the onset, but it completely resolved after medication. He had not dysmorphic facial features. He insisted on maintaining an antalgic posture of the left arm. Left shoulder subluxation was identified and reduction was performed successfully, and thereafter shoulder pain improved slightly. Ten days later, he visited the department of orthopedic surgery because of persistent shoulder pain and limited active range of motion (ROM) of the shoulder. A shoulder magnetic resonance imaging (MRI) showed normal findings and he was referred to the department of rehabilitation medicine. His shoulder pain was 8 on the Numeric Rating Scale (NRS). There was atrophy of the deltoid, supraspinatus and infraspinatus muscles, and scapular winging (Fig. 1). On manual muscle test using the Medical Research Council score, shoulder flexors were 2/5, shoulder extensors 1/5, arm abductors 2/5, and arm adductors 2/5. Deep tendon reflexes of left upper extremity decreased and pathologic reflexes were absent. Electrodiagnostic assessment was performed. Sensory nerve conduction study showed normal findings. Motor nerve conduction study of left axillary, musculocutaneous and suprascapular nerve showed low amplitude. Needle electromyography (EMG) revealed denervation potential and reduced motor unit recruitment pattern in deltoid, infraspinatus, biceps brachii, triceps brachii, and extensor carpi radialis muscles (Tables 1–3). Cervical spine MRI showed normal finding (Fig. 2). He was diagnosed as INA and then was informed of a rehabilitation program. The program was started twice a week and consisted of physical modalities for shoulder pain and gentle passive ROM exercise of the shoulder joint. One month later, shoulder pain decreased (NRS 8→6) and strength partially restored (shoulder flexor 3/5, abductor 3/5, adductor 3/5). Thereafter, the program was changed to active ROM exercise, scapular stabilization exercise, and energy conservation strategies. Four months later, shoulder pain lessened (NRS 6→2), scapular winging diminished, and the strength of shoulder girdle muscles was restored to 4/5, although atrophy of the affected muscles remained. He was able to confidently resume his activities with minimal pain. The patient’s parent provided written informed consent for the publication of clinical details and images.
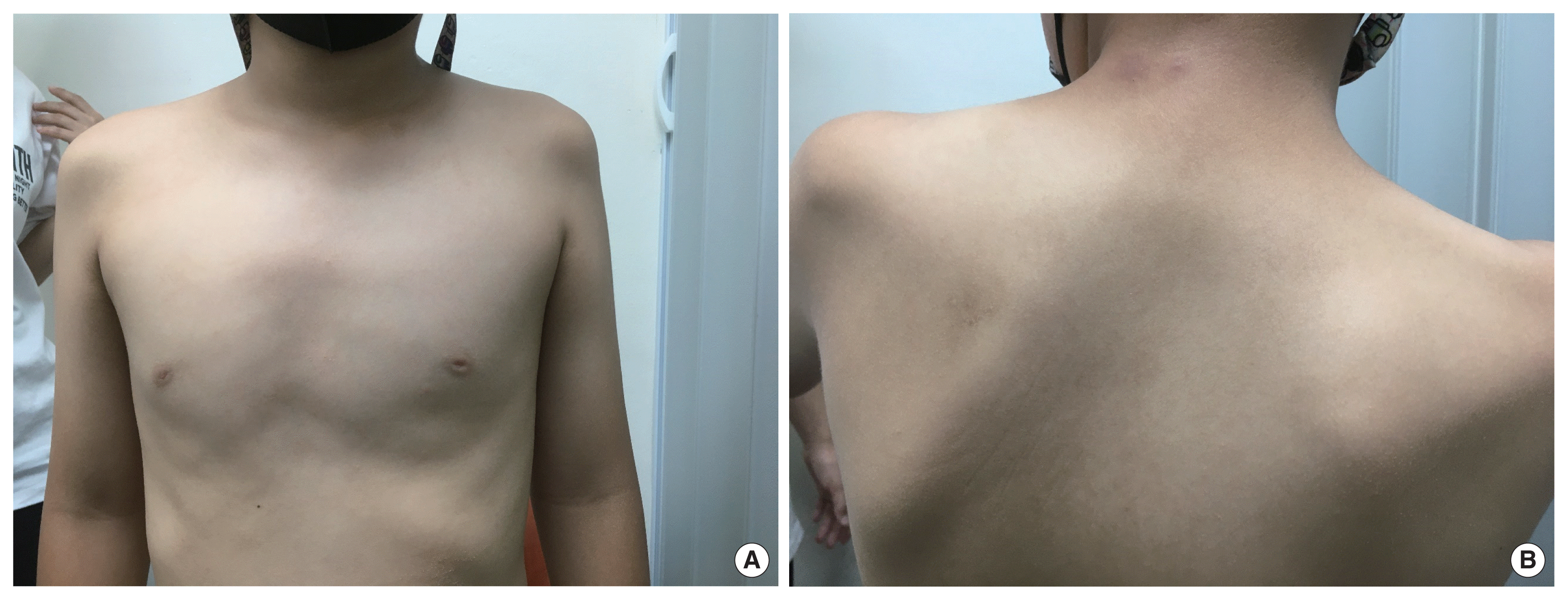
(A) The child shows atrophy of the left deltoid muscle and uneven shoulder. (B) The child shows and marked atrophy of the left supraspinatus and infraspinatus muscle and scapular wining during anterior flexion. Written informed consent for the publication of this image was obtained from the patient.
DISCUSSION
NA is a rare syndrome with various clinical phenotypes and diverse predisposing factors. It manifests in two forms; idiopathic or hereditary. INA frequently occurs after fever, upper respiratory infections (URI), or vaccination, suggesting an immune-mediated pathogenesis [1,6]. HNA is 10 times less common than INA and an autosomal dominant condition characterized by point mutation or duplication in the septin-9 gene on chromosome 17 [9]. Incidence of NA in children varies according to age and has a biphasic pattern with a first sharp peak at newborns (<8 weeks old) and a second broader peak in adolescence (7–15 years old). The present case belongs to the second peak period [3].
The etiology of NA is not clearly identified and several events are believed to act as a triggering factor including fever, vaccination, infection, trauma, exercise, and so forth [3,4,9]. Among those, infection and exercise are more frequent than others [1]. Two events are presumed to have played a role as triggering factors in this case.
The typical clinical course of NA proceeds in three sequential phases: an abrupt onset of severe pain (NRS ≥7/10) around the shoulder or scapular region, paresis with subsequent muscular atrophy and neuropathic pain, and finally recovery or residual musculoskeletal-type pain [1].
Muscle weakness mostly appears within 2 weeks after onset of pain. The muscles innervated by the upper part of the brachial plexus is most commonly involved. Shoulder girdle muscle weakness leads to shoulder subluxation, altered biomechanical change or dysfunctional compensating posture, further causing recurrent muscular strain, musculoskeletal type pain and decreased endurance [6,7]. In addition, the ROM of the shoulder joint is often limited [2].
Not having a confirmative test, classic NA is often diagnosed with both a definite triggering event and a typical clinical course [9]. Owing to its rarity, many studies are usually performed to rule out other diseases. Among those, electrodiagnostic assessment is usually performed to attest the presence of peripheral neuropathy and the severity of axonal injury as well as estimate prognosis [1,5]. Nerve conduction studies (NCS) can reveal axonal injury through decreased amplitude of sensory nerve action potentials (SNAP) or compound motor action potentials in the weak muscles. Unfortunately, SNAP often reveal normal findings despite presence of sensory symptoms [10]. This case showed normal SNAP and therefore a cervical spine MRI was performed to rule out cervical root lesion. Needle EMG is more sensitive than NCS for showing acute denervation and chronic regeneration of individual nerves in paretic muscles [6,10].
Genetic evaluation is needed to differentiate HNA from INA if a patient has clinical features of HNA, such as family history, dysmorphic facial features, and non-classic NA [6,9]. We did not perform a genetic study. However, this was the patient’s first attack and none of the characteristics of HNA was present, making a diagnosis of INA possible.
The standard treatment protocol of INA in children is not established. Some treatments are suggested depending on the clinical phase and features. Within the first month after onset, oral prednisolone is recommended, which can reduce the duration of the initial severe pain and induce earlier recovery of strength [1,3,6]. However, steroid therapy is not frequently initiated owing to the delay of diagnosis as here [10]. The other study suggested a multidisciplinary rehabilitation program according to patient’s needs and goals. The program was shown to improve participants’ functional capability with minimal pain [8]. In that study, however, the participants were older than 18 years and had more than 8 weeks after onset. Although this case did not match the condition of that study, an integrated rehabilitation program was applied. At first, the patient was informed self-management methods for pain control and performed gentle passive ROM exercise. As muscle power improved, active ROM exercise and scapular stabilization exercise were performed gradually. Also, self-care activities training was added to encourage his activities while preventing fatigue and minimizing pain. Although the rehabilitation program might not have a direct effect on the recovery of muscle strength and endurance, it showed to improve the performance and participation in daily activities.
The prognosis of NA in children is variable. It is worse than adults, which is partly due to the delay of early active treatment such as immune therapy owing to lack of clinical experience and delay of the proper diagnosis [4,10]. Another study showed that 88% of pediatric patients demonstrated full or partial recovery and a mean period of recovery was 11.1 months. The reasons of such a different result among studies may be due to a short follow-up period or absence of follow-up in children showing good recovery [3]. In the present study, the patient’s symptoms improved greatly within 6 months after the onset, although he had not received immunotherapy. This seems to be somewhat related to the partial extent of brachial plexopathy on the electrodiagnostic assessment because partially injured nerve recovery proceeds by collateral reinnervation and takes several months [6].
In conclusion, the author suggested that an integrated rehabilitation programs is helpful for the facilitating functional recovery and reducing the severity of sequelae in a child diagnosed as INA, if it is applied during subacute phase of the disease. If more cases and long-term follow-up are accumulated later, the effect of the rehabilitation program can be more accurately identified.
Notes
No potential conflict of interest relevant to this article was reported.