The Coexistence of Fibromuscular Dysplasia of the Renal Artery and Graves’ Disease in an Adolescent: A Case Report
Article information
Abstract
Here we present a rare case of an adolescent with hypertension, concurrently diagnosed with fibromuscular dysplasia of the renal artery and Graves’ disease. Although fibromuscular dysplasia and Graves’ disease have distinct pathogenic mechanisms, it is possible to infer the potential correlation between the two from the perspective of vascular involvement. It is believed that transforming growth factor-β, as the shared element of both diseases, may contribute to their development and progression. The overactivation of the sympathetic nervous system in Graves’ disease may induce hyperplasia of vascular smooth muscle cells, similar to that observed in fibromuscular dysplasia. In Graves’ disease, the excessive synthesis and secretion of angiotensin II due to the overactivation of the renin-angiotensin system, along with the up-regulation of angiotensin II receptors, may also induce pathological changes in the vasculature throughout the body. In this regard, exploring the correlation between fibromuscular dysplasia and Graves’ disease is of significant clinical importance.
INTRODUCTION
The coexistence of fibromuscular dysplasia and Graves’ disease is exceedingly rare and their precise pathogeneses remain unclear [1,2]. Fibromuscular dysplasia and Graves’ disease are considered separate diseases, but when simultaneously diagnosed, their association warrants consideration. Herein, we report a rare case of an adolescent who was concurrently diagnosed with fibromuscular dysplasia of the renal artery and Graves’ disease. We explore the possibility of an association between these two conditions in terms of their pathogenesis and progression with a literature review.
CASE REPORT
A 13-year-old female presented with hypertension without headaches, dizziness, palpitations, or chest discomfort. The patient had a family history of hypertension reported from the grandmother. Physical examination revealed mild eyeball protrusion and moderate goiter without tenderness. Additionally, systolic murmurs were detected upon auscultation. Repeated blood pressure (BP) measurements during the visit consistently showed greater than 160/90 mm Hg (systolic blood pressure [SBP]/diastolic blood pressure [DBP]), which indicated stage 2 hypertension according to American Academy of Pediatrics guideline in 2017 (SBP >129 mm Hg, DBP >77 mm Hg). Initial blood tests revealed normal: white blood cell, hemoglobin, platelet, erythrocyte sedimentation rate, C-reactive protein (CRP), blood urea nitrogen, creatinine, electrolytes, liver function tests, lipid, rheumatoid factor, C3, C4, antineutrophil cytoplasmic antibodies, and antinuclear antibodies. Elevated levels of triiodothyronine (T3) at 317 ng/dL (normal range, 80–200 ng/dL) and free thyroxine (fT4) at 3.64 ng/dL (normal range, 0.93–1.70 ng/dL) with suppressed thyroid-stimulating hormone (TSH) at 0.005 μIU/mL (normal range, 0.7–6.4 μIU/mL) were checked. Additionally, thyroid autoantibodies were elevated, with antithyroglobulin (thyroid peroxidase) antibodies at 259.5 IU/mL (normal range, 0–115 IU/mL) and TSH receptor antibodies at 6.13 IU/L (normal range, 0–1.75 IU/L). Conclusively, the patient was diagnosed with Graves’ disease. Moreover, 24-hour BP monitoring revealed stage 2 hypertension with non-dipper status as the following average BP results (systolic/diastolic): 175/119 mm Hg during activity, 186/132 mm Hg during sleep, and an overall average of 177/121 mm Hg. Given that the SBP and DBP exceeded the 99th percentile, further investigations were conducted to exclude other causes of secondary hypertension. Angiotensin-converting enzyme levels were within normal limits, but plasma renin activity was elevated at 8.43 ng/mL/hr compared to the age-specific normal range of 1.6–5.4 ng/mL/hr. The concentrations of epinephrine, norepinephrine, and dopamine in the 24-hour urine were also within the normal range. Mild left ventricular hypertrophy was observed on echocardiography, and renal computed tomography (CT) angiography revealed a stenosis at the proximal part of the left renal artery with a 1.6-cm aneurysm (Fig. 1). Further CT angiography of the aorta and brain revealed no additional stenosis or aneurysms. A captopril renal scan showed a decrease in left kidney function after captopril administration, considering the possibility of renin-dependent renovascular hypertension. Thyroid ultrasonography did not identify any thyroid nodules, but a Tc-99m pertechnetate thyroid scan revealed an enlarged thyroid gland with increased uptake throughout (Fig. 2). Funduscopic examination showed no hypertensive retinopathy. Based on the above test results, the patient was diagnosed with coexisting Graves’ disease and renal artery stenosis.

Renal computed tomography angiography. There are the concentric stenosis of the left main renal artery and the post-stenotic aneurysm sized 1.6 cm with posterior branch arterial tubular stenosis from the aneurysm.
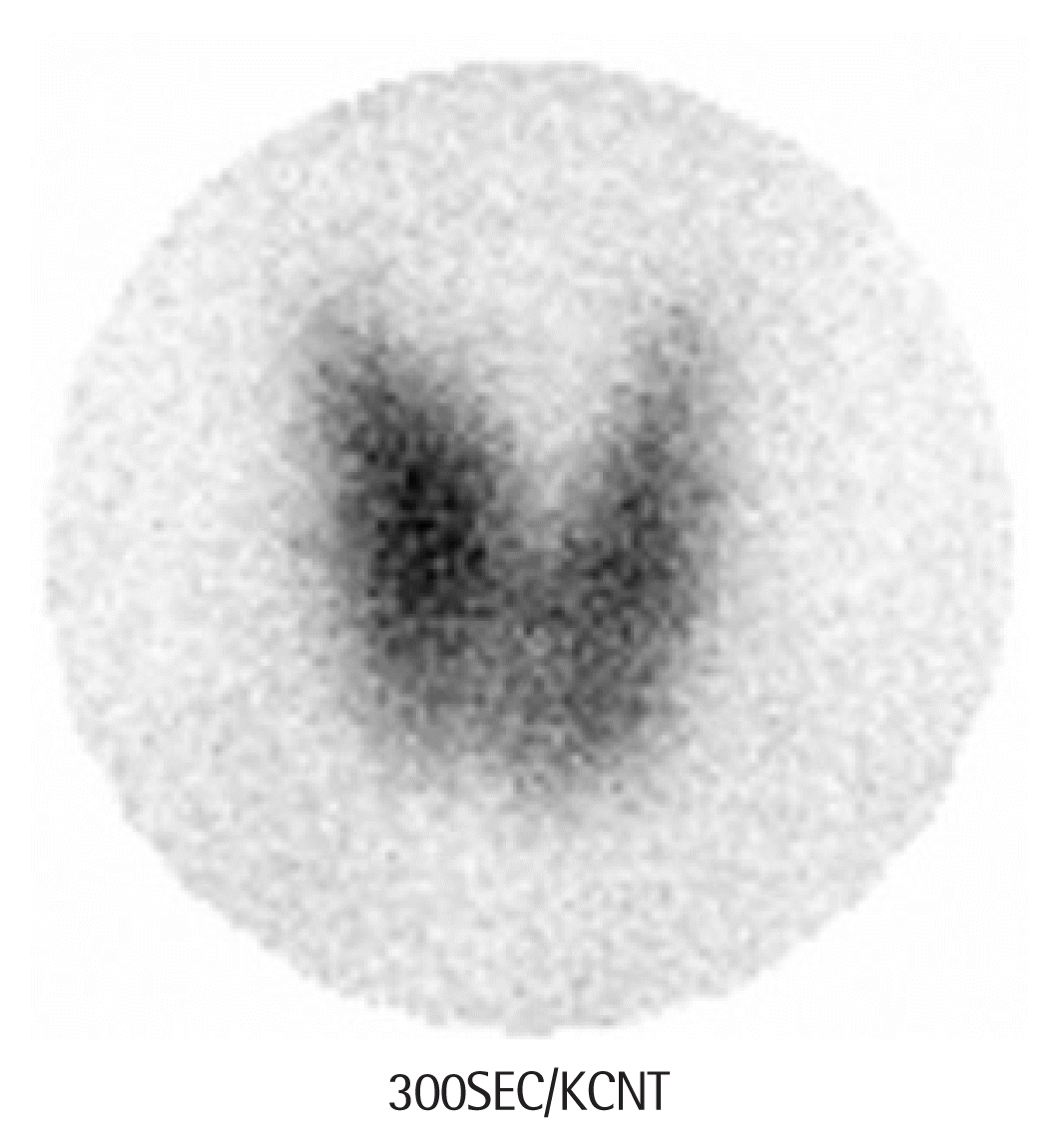
Tc-99m pertechnetate thyroid scan. Diffusely enlarged thyroid with increased uptake of Tc-99m pertechnetate is checked.
Considering the possible causes of renal artery stenosis, a genetic etiology was not suggestive because the patient had no history of developmental delay and a familial predisposition to hereditary disorders. Imaging did not reveal any externally compressed lesions, ruling out stenosis caused by mechanical compression. Vasculitis was considered unlikely due to the patient’s lack of symptoms, no specific examination results, normal inflammatory markers, and no additional stenosis and aneurysms in other arteries. However, it is difficult to completely exclude the possibility of inflammatory changes in the renal artery due to an immune response, considering the coexistence of Graves’ disease. Clinically, fibromuscular dysplasia was considered highly likely, and the plan was to perform tissue biopsy for histological confirmation during renal angiography and angioplasty. Before intervention, considering the possibility of vasculitis and fibromuscular dysplasia, the patient was started on oral medications including prednisolone and azathioprine for vasculitis, amlodipine for hypertension, and methimazole and propranolol for Graves’ disease. During follow-up, the patient’s medication compliance was somewhat suboptimal, resulting in an average BP decrease to 125/85 mm Hg, which was still greater than the normal range. The T3 and fT4 levels normalized, while TSH remained lower than the normal limit. The inflammatory markers remained consistently within the normal range and renal CT angiography revealed the stable conditions of the stenosis and aneurysm in the left renal artery. Therefore, prednisolone and azathioprine for vasculitis were discontinued. Even after discontinuing them, the level of the inflammatory markers remained normal, and the vascular lesions showed no progressive changes in the CT scan. Clinically, the possibility of fibromuscular dysplasia was considered to be higher. Prior to the biopsy, the patient has been continuing observation and medication (amlodipine, methimazole, and propranolol), as her BP and thyroid functions remain acceptable and no progression of fibromuscular dysplasia is revealed. Requirement for informed consent was waived after review of IRB because this study was retrospective design and was practically impossible to obtain consent from the study subjects.
DISCUSSION
The precise pathogenesis of fibromuscular dysplasia and Graves’ disease remains poorly understood [1,2]. Fibromuscular dysplasia is a non-atherosclerotic disease that affects medium-sized arteries in the body. It is believed to result from a complex interplay of genetic factors, such as single-nucleotide polymorphisms of PHACTR1, and environmental factors, including increased progesterone receptor expression in vascular smooth muscle cells and smoking history [1,3]. Graves’ diseases is an autoimmune disorder resulting from an imbalance in T-cell differentiation and function and can lead to vascular complications [2]. Although fibromuscular dysplasia and Graves’ disease have independent etiological theories, when diagnosed concurrently, consideration should be given to the correlation of the occurrence and progression between Graves’ disease and fibromuscular dysplasia. The pathophysiological characteristics of each condition can provide insights into their potential association.
Recent studies have suggested that fibromuscular dysplasia exhibits inflammatory progression during its early stages, challenging the main characteristic as a “non-inflammatory condition” and instead postulating its characterization as an “inflammatory systemic disease” [1,3]. Studies on patients with fibromuscular dysplasia have shown elevated levels of inflammatory markers, such as CRP and tumor necrosis factor-α with increased stiffness of unaffected arteries [1,3,4]. These findings suggest the presence of inflammatory processes in fibromuscular dysplasia, which can impact systemic arteries [1,3,4]. Transforming growth factor-β (TGF-β) has been identified as a likely participant in the inflammatory process of fibromuscular dysplasia [1,4]. It has been confirmed that the levels of TGF-β in the serum of patients with fibromuscular dysplasia, along with the secretion of TGF-β1 and β2 from their dermal fibroblasts, are increased [1,3,4]. In fibromuscular dysplasia, TGF-β which can induce arterial remodeling may indirectly influence arterial stenosis by interacting with inflammatory processes involving interleukin (IL)-17 and IL-9, leading to fibrosis [1,4]. In addition to arterial stenosis, TGF-β1 may inhibit secretion of IL-8, which is thought to play a pivotal role in endothelial cell survival and proliferation, potentially acting as a contributing factor to arterial aneurysms [4]. Moreover, the action of TGF-β has implications for the etiology of Graves’ disease. TGF-β alone induces the differentiation and proliferation of regulatory T cells (Treg), inhibits the activation of thyroid follicular cells and suppresses the generation of antibodies by B cells and the secretion of pro-inflammatory cytokines, thus acting as a suppressor of autoimmune reactions [5,6]. However, the simultaneous effects of TGF-β and IL-6 during CD4+ T-cell differentiation may induce the differentiation of helper T 17 cells, which secrete inflammatory cytokines (IL-17, IL-21, IL-22) and suppress Treg differentiation, thus playing major roles in autoimmune responses [5,6]. These findings suggest that TGF-β may serve as a common factor connecting the pathogenesis of fibromuscular dysplasia and Graves’ disease.
Graves’ disease induces hypertension by stimulating the sympathetic nervous system, leading to vascular complications [7,8]. These can occur because of the activation of postganglionic sympathetic nerve cells, inducing the proliferation of vascular smooth muscle cells [8]. Damon [8] demonstrated that co-culture of a rat superior cervical ganglion with an aorta induced the activation of postganglionic sympathetic nerve cells, leading to the formation of new synapse connections, thereby promoting the proliferation of aortic smooth muscle cells. In this process, TGF-β2 acts as a mediator [8]. Furthermore, overactivation of the sympathetic nervous system can influence the systemic vasculature, indicating that smooth muscle cells in renal arteries could be proliferated under the control of the aorticorenal ganglion. Regarding the role of TGF-β2 as a mediator in this process, additional research is required to ascertain its potential extension for interpreting its association with fibromuscular dysplasia.
Additionally, Graves’ disease induces overactivation of the renin-angiotensin system, leading to inflammatory responses through increased angiotensin II activity [7,9]. Angiotensin II can induce the proliferation of vascular smooth muscle cells, and T3 can enhance the expression of angiotensin II receptors, increasing the sensitivity to angiotensin II [7,8,10]. Angiotensin II also has pro-inflammatory effects contributing to vascular pathogenic changes by increasing vascular permeability, initiating vascular inflammatory changes, and inducing fibrosis [10]. This inflammatory action of angiotensin II is also noteworthy during the early stages of fibromuscular dysplasia [1]. Therefore, further research is required to elucidate the direct influence of Graves’ disease-induced angiotensin II activation on the development of fibromuscular dysplasia.
This case provides valuable insights into a novel etiological approach concerning the potential association between fibromuscular dysplasia and Graves’ disease. However, cohort studies on patients with both conditions and pathophysiological investigations into their direct relationship are currently lacking. Therefore, further research is warranted to explore the correlation between these two conditions.
Notes
No potential conflict of interest relevant to this article was reported.