Bone Biopsy Confirmed Imatinib-Induced Bone Marrow Necrosis in Patient with Gastrointestinal Tumor: A Case Report
Article information
Abstract
Imatinib mesylate is an effective, small-molecule, selective tyrosine kinase inhibitor, which inhibits BCR-ABL in patients with chronic myeloid leukemia, c-kit in patients with gastrointestinal tumor (GIST), and platelet-derived growth factors in hypereosinophilic syndrome. Above all, bone marrow necrosis is a rare complication of imatinib usage. If newly developed increased opacity bone lesions are seen in patients with metastatic GIST, it is usual to consider those lesions as bone metastasis. Also, it is true that making a differential diagnosis between bone marrow change (bone marrow necrosis) and disease progression (bone metastasis) is both clinically and radiologically difficult. Because it may alter treatment options according to early distinguish bone metastasis and imatinib related-bone necrosis, a bone biopsy is recommended. Here, we report a case that a biopsy confirmed bone marrow necrosis due to imatinib, even though investigations such as magnetic resonance imaging, and whole-body bone scan indicated more favor to the metastatic lesion.
INTRODUCTION
Imatinib mesylate acts by inhibiting selective tyrosine kinase signal transduction, it has become the worldwide front-line standard treatment of malignant diseases such as chronic myeloid leukemia (CML), hypereosinophilic syndrome (HES) and gastrointestinal tumor (GIST) [1]. In regard to adverse events, the spectrum of hematologic adverse reactions possibly due to imatinib mesylate therapy reported range from anemia, neutropenia [2], thrombocytopenia, purpura, hematoma, and myelosuppression [3]. Especially for GIST, it is strongly associated with c-kit mutation; imatinib as a small molecule tyrosine kinase inhibitor blocks those c-kit mutations. Then imatinib is generally tolerated well by patients with metastatic GIST, severe adverse events are rare, including bone-related lesions [4]. However, a few papers have been published, which are indicating imatinib usage-related bone marrow necrosis [4–6]. At this present, it is true that making a differential diagnosis between bone marrow change (bone marrow necrosis) and disease progression (bone metastasis) is both clinically and radiologically difficult. We report one case of focal bone marrow abnormalities detected on whole body bone scan and magnetic resonance (MR) imaging that appeared in the sacral bone of a patient who was responding to imatinib.
CASE REPORT
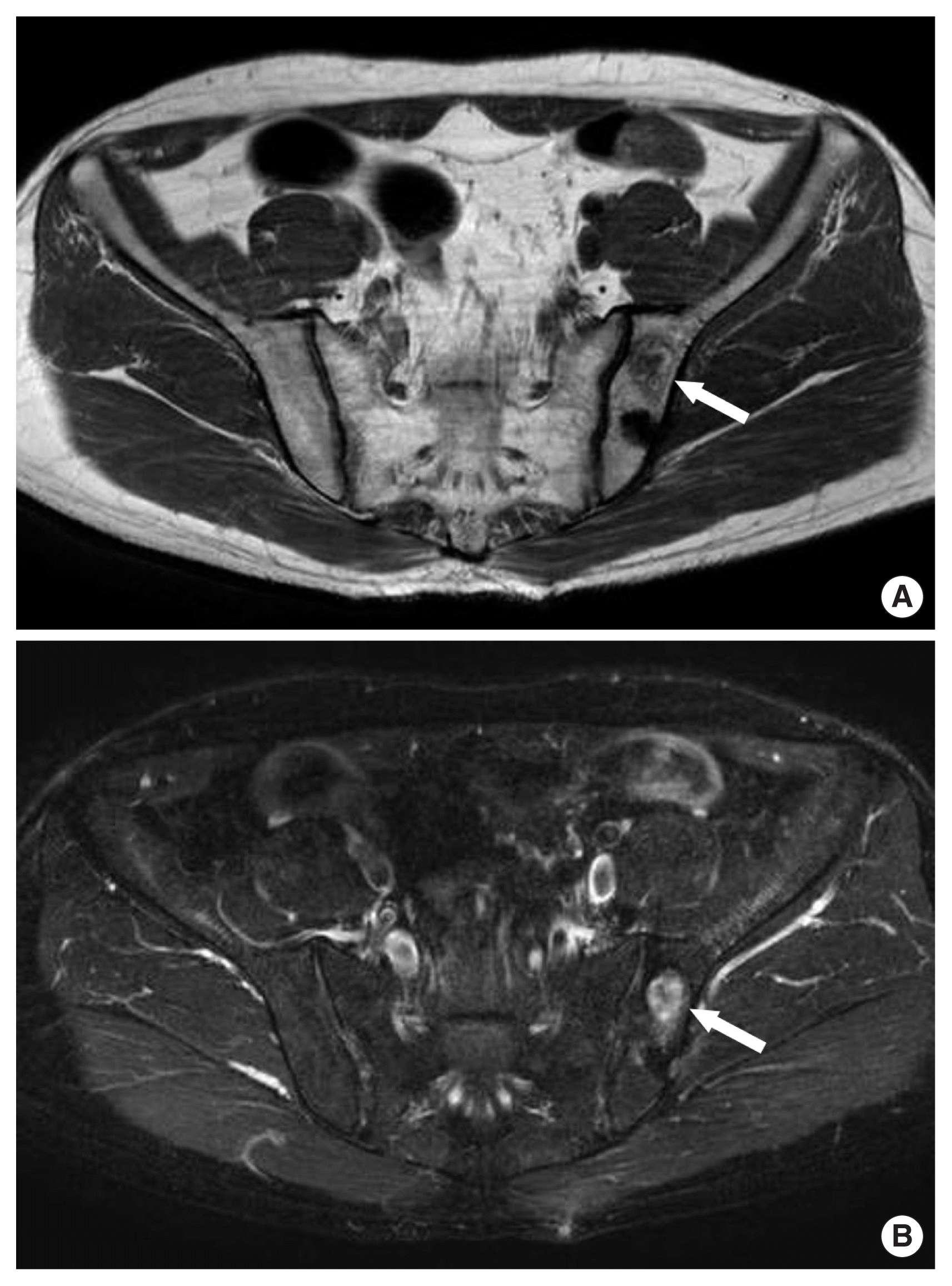
A 50-year-old man was transferred with intermittently nonspecific pain in the left pelvic area. He had a past history which was wedge resection of the stomach and segmental resection of the transverse colon due to a metastatic malignant GIST 4 years ago. Because pathologic findings showed the high mitotic activity of the tumor and metastasis, he was still undergoing maintenance chemotherapy (400 mg imatinib per day). The serial computed tomography (CT) scan had been shown no abnormal findings. All laboratory findings were within normal range including alkaline phosphatase and lactate dehydrogenase, except for normocytic normochromic anemia as 10.5 g/dL of hemoglobin. And other tumor markers were within normal range. Plain radiographs of the pelvic bone showed normal findings. However, in comparison to the previous image of the whole-body bone scan (Fig. 1A), a newly developed increased radioiodine uptake lesion is seen in the left sacral ala on whole body bone scan (Fig. 1B). Furthermore, MR imaging of the pelvic bone revealed suspicion of a mass-like lesion (2.7×1.3×2.3 cm sized) on left iliac bone with low signal intensity on T1-weighted image (Fig. 2A) and high signal intensity on T2-weighted image (Fig. 2B), with heterogeneous contrast enhancement (it showed the enhancement of rim, but no enhancement of inner space). Screening for tuberculosis including Mantoux (PPD [purified protein derivative]), rapid mycobacterium culture (BACTEC), and acid-resistant basal tests and serum QuantiFERON levels were normal. Although imaging studies showed favor for metastatic lesions, we decided to perform the pelvic bone biopsy to distinguish between metastatic lesions and other causes of necrosis. The final pathologic report of the biopsy specimen indicated hypocellular marrow and necrosis with osteomyelitis, no evidence of malignancy, and a negative c-kit on immunohistochemistry staining. After 3 weeks of inpatient care, the patient was discharged from the hospital with no significant events. He fared well for 2 years without any bone problems, before developing general resistance to imatinib. The patient provided verbal consent for the publication of clinical details and images.

Whole-body bone scan image. (A) Previous serial image of whole-body bone scan: there is no evidences of abnormalities of entire skeletal systems (white arrow). (B) Whole-body bone scan for restaging workup: newly developed increased radioiodine uptake lesion is seen in left sacral ala (black arrow).
DISCUSSION
Imatinib mesylate is an effective, small-molecule, selective tyrosine kinase inhibitor, which inhibits BCR-ABL in patients with CML, c-KIT in patients with GIST, and platelet-derived growth factors in HES [1,7]. Imatinib has become the treatment of choice for metastatic GIST. In the era of imatinib usage for GIST, survival rates for metastatic GIST have improved, with nearly 90% of patients achieving a 2-year survival rate of 75% [7]. While the drug is generally well tolerated in the majority of patients, the increasing use of imatinib in mentioned diseases has led to a significant number of reported hematologic complications, including anemia, thrombocytopenia, neutropenia [2], pancytopenia, and autoimmune hemolytic anemia [3]. Most of all, therapy-induced bone marrow necrosis has been reported recently in patients with CML treated by imatinib [6,8]. It seems that the dosage and duration of imatinib play a role in the expression of bone marrow aplasia and necrosis [9]. Although the mechanism leading to bone marrow necrosis with imatinib therapy is not yet clear, bone marrow necrosis is due to cellular hypoxia caused by inflammatory damage or mechanical obstruction of bone marrow microcirculation [4]. The increased rate of prothrombotic cellular material release and increased rate of apoptosis have been believed to be responsible [4]. But, in patients with malignancy, if newly developed increased opacity bone lesions are seen, it is usual to consider those lesions as bone metastasis. In recent, imatinib related-bone marrow necrosis mimicking metastasis on MR imaging [10], and bone marrow necrosis secondary to imatinib usage, mimicking spinal metastasis on MR imaging [11] have been reported. According to Aras et al. [11], making a radiological distinction between these two pathologies is not always possible. A tissue biopsy confirmation is indicated for differential diagnosis, although whole body bone scan, MR imaging, and CT scan could help to distinguish imatinib-induced bone necrosis and bone metastasis. With increasing imatinib usage and improved survival rates in metastatic GIST, new problems such as bone marrow necrosis occur. Thus, because this condition is radiologically and clinically similar to metastasis, physicians should pay particular attention to distinguishing imatinib-induced bone necrosis from bone metastasis, which may alter treatment options, and then more conservative methods such as percutaneous or endoscopic bone biopsies are recommended including imaging studies.
In conclusion, even if bone marrow necrosis due to imatinib is rare, it is not easy to distinguish it from bone metastasis. So, if the patients with high doses and a long duration of imatinib usage have bone pain or bone fractures, physicians should consider disease progression (metastasis) and imatinib-induced bone necrosis as well. And then a bone biopsy is recommended.
Notes
No potential conflict of interest relevant to this article was reported.