Early Postoperative Pulmonary Edema Attributable to Reverse Stress-Induced Cardiomyopathy in a Recently Infected COVID-19 Patient: A Case Report
Article information
Abstract
The coronavirus disease 2019 (COVID-19) pandemic has increased the incidence of stress-induced cardiomyopathy (SICMP). A 33-year-old woman without any notable medical history underwent an emergency operation to treat a ruptured ectopic pregnancy. She entered hemorrhagic shock attributable to massive bleeding of the ruptured ectopic sac, followed by rapid transfusion and hydration, and vasopressor therapy. Her COVID-19 rapid antigen test was negative before surgery. After surgery, her vital signs were stable and she was mentally alert. However, about 1 hour later, she developed pulmonary edema, was re-intubated, and was admitted to the intensive care unit. There, echocardiography revealed reverse SICMP, and a COVID-19 polymerase chain reaction test was positive. She recovered well on conservative treatment. After 9 days, her echocardiography profile was normal and she was discharged without any cardiac symptoms or complications. Anesthesiologists should be aware that COVID-19-infected patients may develop postoperative SICMP.
INTRODUCTION
Stress-induced cardiomyopathy (SICMP) is characterized by acute, temporary, left ventricular wall motion abnormalities in patients lacking significant coronary disease [1]. Perioperative SICMP attributable to emotional or physical stress imposed by surgery has been reported [2]. Post-procedural SICMP is associated with more mortality than simple emotional SICMP [2]. The coronavirus disease 2019 (COVID-19) pandemic imposes social and emotional stresses that can cause SICMP, in addition to other medical problems. The incidence of SICMP has increased 3–4-fold in the COVID-19 era [1]. The link between COVID-19 infection and SICMP features the usual increases in psychological distress, a cytokine storm, increased sympathetic responses, and microvascular dysfunction [1]. The cytokine storm, angiotensin-converting enzyme 2 deficit, and proteoglycan breakdown render COVID-19-infected patients vulnerable to pulmonary edema [3]. Here, we describe a case of early postoperative pulmonary edema attributable to SICMP in a patient with a recent COVID-19 infection not detected preoperatively by the rapid antigen test.
CASE REPORT
A 33-year-old, 56.1-kg, 160-cm-tall woman underwent emergency left salpingo-oophorectomy to treat a ruptured ectopic pregnancy. She had no notable medical or surgical history, particularly no history of chest pain. She had received three doses of the COVID-19 vaccine and her rapid antigen test was negative. Her initial vital signs included a non-invasive blood pressure of 85/57 mm Hg and a heart rate of 92 bpm. The preoperative laboratory test results were within the normal ranges; the hemoglobin (Hb) level and hematocrit (Hct) were 11.1 g/dL and 32.7%, respectively. Her cardiac marker levels were within the normal ranges (creatine phosphokinase-MB [CK-MB], 0.77 ng/mL; troponin T, <0.003 ng/mL). An electrocardiogram and chest radiograph were unremarkable (Figs. 1, 2). She claimed that she could not be pregnant, but her urine pregnancy test was positive and her serum beta-human chorionic gonadotropin level was 15,161.8 mU/mL (non-pregnant: 0–5 mU/mL). Within 1 hour, her non-invasive blood pressure measurement became 67/41 mm Hg, her heart rate was 110 bpm, and her Hb/Hct readings fell from 11.1 g/dL/32.7% to 5.9 g/dL/17.5%. Emergency transthoracic echocardiography revealed normal left ventricle contractility and a normal right ventricle strain without any pericardial effusion. However, the inferior vena cava diameter was less than 2 cm and evidenced inspiratory collapse; we diagnosed hypovolemic shock. Abdominal ultrasonography revealed a large hematoma. We thus transfused two units of packed red blood cells (pRBCs), administered tranexamic acid (1,000 mg), and commenced a norepinephrine infusion at 0.15 mcg/kg/min. After 30 minutes, her non-invasive blood pressure was 41/22 mm Hg and her heart rate was 40 bpm. The norepinephrine was increased to 0.3 mcg/kg/min and atropine (1 mg) was administered. The transfusion was completed 10 minutes later. She was taken to the operating room (OR) and transfused with an additional two units of pRBCs.
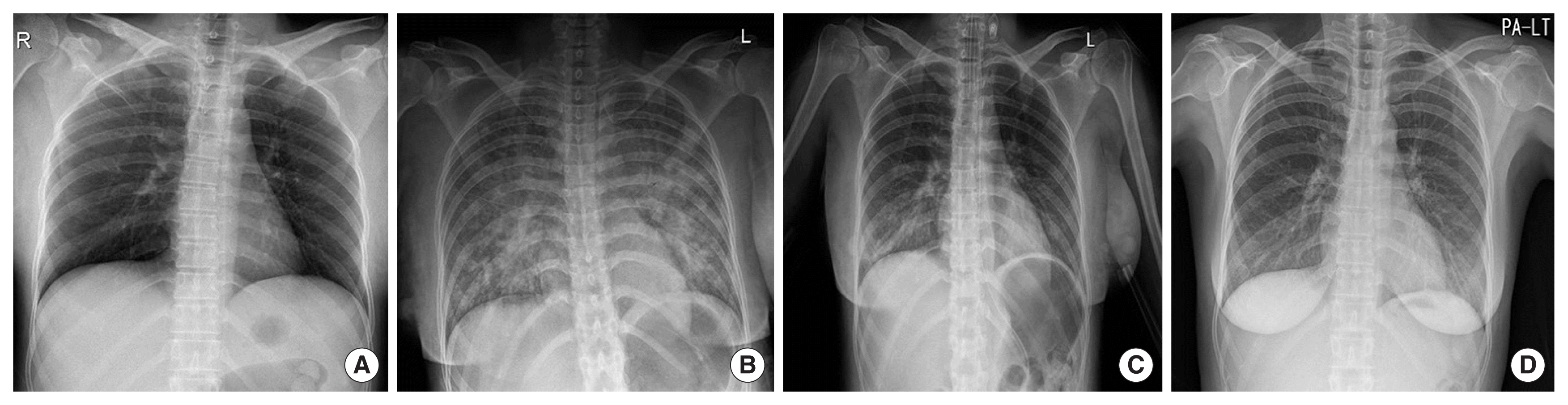
The perioperative chest X-rays. (A) A preoperative anterior-posterior chest X-ray was normal on arrival in the emergency room. (B) The anterior-posterior chest X-ray taken in the post-anesthesia care unit. Pulmonary edema is evident in both lungs and the oxygen saturation fell to 40%. (C) The anterior-posterior chest X-ray taken 1 day after surgery. The edema is improving in both lungs. (D) The posterior-anterior (PA) chest X-ray taken 9 days after surgery. The increased shading of both lower lobes may indicate post-coronavirus disease 2019 pneumonia.
Perioperative electrocardiograms. (A) The preoperative electrocardiogram taken at admission. The sinus rhythm was normal on arrival in the emergency room. (B) The postoperative electrocardiogram taken at arrival in the intensive care unit. Sinus tachycardia is apparent. (C) The electrocardiogram taken 3 days after surgery. The sinus rhythm is normal.
In the OR, the patient’s initial non-invasive blood pressure was 154/112 mm Hg and her heart rate was 140 bpm. Anesthesia induction and intubation were facilitated with lidocaine, propofol (40 mg), and rocuronium (50 mg). After intubation, her lung sounds were clear. Anesthesia was maintained with desflurane in 40% oxygen, a brachial arterial cannula was placed, and the operation commenced. The vital signs were unstable given the hemorrhagic shock caused by massive blood loss; two units of pRBCs and 500 mL of colloid (Volulyte, 6% weight per volume [w/v]; hydroxyethyl starch [HES], 130/0.4) were rapidly administered to fill the circulatory volume. The patient’s urine output and arterial blood gas profile were monitored in real time to regulate the fluid balance. Also, 300,000 IU of ulinastatin (Ulistin; Hanlim Pharmaceutical, Seoul, Korea) was administered (an anti-inflammatory treatment for shock). Hemodynamic management during anesthesia featured norepinephrine administration to maintain a mean blood pressure of 60–70 mm Hg. The stroke volume variation (<10%) and cardiac index (>3.0 L/min/m2) were monitored and maintained at normal values using a FloTrac device (Vigileo; Edwards Lifesciences, Irvine, CA, USA). Laparoscopy revealed a 7-mm-sized rupture of a gestational sac and about 4 L of blood and hematoma in the abdominal cavity. Intraoperatively, she was transfused with two units of pRBCs, 1.5 L of colloid (Volulyte, 6% w/v; HES, 130/0.4), and 600 mL of crystalloid (a plasma solution). At the end of the surgery, the patient’s urine output was 50 mL, and her Hb level was 11.5 g/dL by arterial blood gas analysis. The total anesthesia time was 85 minutes. Sugammadex (200 mg) was used to reverse the neuromuscular blockade. After confirming that she was alert and breathing spontaneously, she was extubated and transported to the post-anesthesia care unit (PACU).
In the PACU, she remained alert, her breathing sounds were clear, and the arterial mean blood pressure of 65–70 mm Hg persisted on norepinephrine administration. Within 1 hour in the PACU, she met the Modified Aldrete score criterion and was prepared for discharge to a general ward. However, her oxygen saturation suddenly fell to 40% and she complained of shortness of breath. We immediately attached a non-rebreathing circuit. Crackling sounds were evident on auscultation and an emergency chest X-ray revealed edema in both lungs (Fig. 1). She was intubated and the breathing assistance intensified. A large amount of pinkish liquid was ejected through the endotracheal tube; endotracheal suction was performed every minute. Her blood pressure fell; the norepinephrine dose was increased and furosemide (10 mg) was administered. She was again transported to the intensive care unit (ICU). On arrival, her arterial blood gas parameters were as follows: pH, 7.25; partial pressure of carbon dioxide (PCO2), 42.9; partial pressure of oxygen (PO2), 65.9; HCO3−, 18.5; base excess (BE), −8.3; and O2 saturation, 89.9.
She was echocardiographically diagnosed with SICMP (ejection fraction [EF]=29%) and hypokinesia of the base of the left ventricle. Her cardiac marker levels increased (CK-MB, 11.15 ng/mL; troponin T, 0.437 ng/mL; ProBNP, 94 pg/mL) (Fig. 3). To treat the hypotension, intravenous norepinephrine was maintained and appropriately adjusted. Mechanical ventilation was initiated to treat acute respiratory failure, and furosemide was periodically administered. The next day, a chest X-ray revealed that the pulmonary edema had improved and arterial blood gas analysis indicated that the partial oxygen pressure had normalized (pH, 7.47; PCO2, 26.7; PO2, 182.7; HCO3−, 19.2; BE, −3.2; and O2 saturation, 99.4) (Fig. 1). The cardiac marker levels had fallen (CK-MB, 7.69 ng/mL; troponin T, 0.159 ng/mL). She was extubated and a nasal cannula was placed. The postoperative COVID-19 polymerization chain reaction test was positive and she was admitted to the isolation room; all patients and medical staff with whom she had come in contact were negative. Remdesivir was administered for 3 days. On postoperative day (POD) 7, her blood pressure remained stable and norepinephrine was discontinued. On POD 8, the COVID-19 cycle threshold value was >30 and she was transferred to a general ward. On POD 9, echocardiography was normal (EF=58% and no cardiovascular symptom). However, she complained of a persistent dry cough; a chest X-ray revealed increased shading of both lower lobes (Fig. 1). Post-COVID pneumonia was suspected, but the symptoms were not severe and she was discharged. Four days later, she visited our outpatient clinic with no respiratory symptoms. On that day, she gave us written informed consent for the publication of her case.
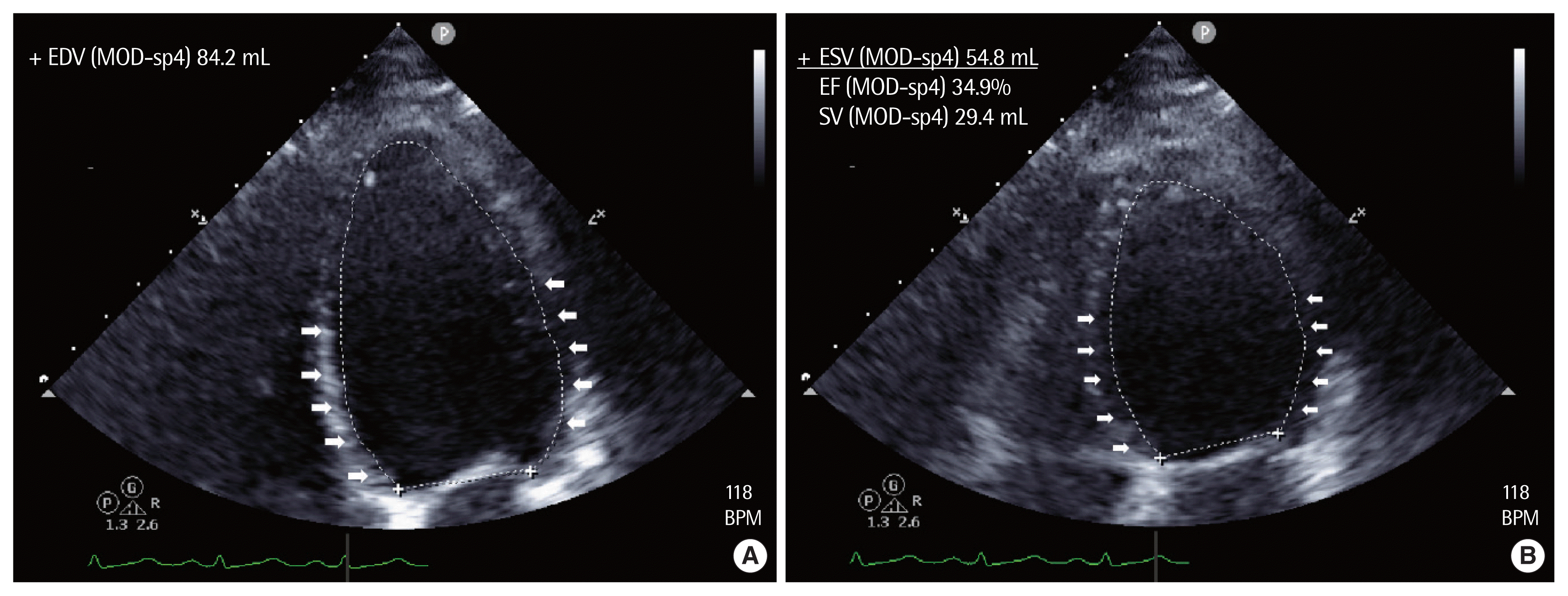
Postoperative transthoracic echocardiography. Echocardiography performed on arrival in the intensive care unit revealed basal hypokinesia of the left ventricle. (A) End diastole (arrows). (B) End systole (arrows; four-chamber views). EDV, end-diastolic volume; ESV, end-systolic volume; MOD, method of disks; sp4, single plane, apical 4 chamber; EF, ejection fraction; SV, stroke volume.
DISCUSSION
SICMP is an acute heart disease syndrome caused by emotional and physical stress. The clinical characteristics are similar to those of acute coronary syndrome, but neither significant stenosis nor ruptured atherosclerotic plaque in the coronary artery is apparent [1]. Apical hypokinesia is the most common form of SICMP (62%–88%), but basal hypokinesia has been reported in up to 4.8% of cases (such as our case) [4]. Physical rather than emotional stress is the prime trigger of SICMP, although uneventful surgery may also induce the condition [2]. SICMP caused by physical stress is associated with higher mortality than other forms of SICMP [2]. SICMP case numbers are increasing; the number was about 20-fold greater in 2012 than in 2006 [5]. One large study on postoperative SICMP found that the incidence was 17.74 per 100,000 [6]. The pathophysiology of COVID-19 infection and the various stresses caused by the pandemic have increased the incidence of SICMP [1].
COVID-19 is spreading rapidly worldwide, increasing the prevalence of cardiovascular problems, including SICMP [1]. Social and emotional stress aside, a link between COVID-19 pathophysiology and SICMP has been suggested [1]. First, the cytokine storm of COVID-19 patients may trigger SICMP. During the storm, pro-inflammatory chemokines and cytokines are rapidly delivered to the blood, provoking vascular leakage and epithelial/endothelial cell apoptosis [1]. Second, the catecholamine surge may evoke SICMP by triggering endothelial dysfunction (vasospasm and inflammation), which dysregulates left ventricular function [7]. Last, COVID-19 infection also activates the hypothalamic–pituitary–adrenal axis, inducing hypercortisolism and suppressing corticotropin release. Cortisol excess and adrenal deficiency have been reported in SICMP cases [8].
In our case, several factors, including the aforementioned COVID-19-to-SICMP link, may have triggered SICMP. The various possible factors follow. First, the physical stresses imposed by acute hemorrhagic shock and surgery increase catecholamine levels. In addition, she was infused with norepinephrine (a catecholamine) at high doses; high catecholamine levels induce cardiac stunning, damage cardiac myocytes [1], and trigger SICMP. Also, a hormonal effect may have been in play. A large proportion of postoperative SICMP in young female patients occurs after obstetric and gynecological procedures. Also, SICMP is more frequent in postmenopausal women, suggesting a hormonal relationship [1,9]. Our young female patient underwent obstetric surgery. Finally, the emotional stresses associated with not only the COVID-19 pandemic but also other factors may be relevant. She insisted that she could not be pregnant, but had an ectopic pregnancy, and required emergency surgery. In short, emergency surgery worried her, and her unexpected pregnancy depressed her. Her stress level was very high.
Pulmonary edema developed rapidly within 1 hour of surgery. This may be explained by COVID-19-induced changes in the pulmonary vasculature and/or loss of lung proteoglycans. Also, a neurogenic mechanism may have been in play [10]. Second, rapid administration of large amounts of fluid and pRBCs to deal with the hemorrhagic shock may trigger pulmonary edema. However, in our case, the blood loss (in the suction bottle of the OR) was at least 4 L, thus more than the volume of fluid administered. The body fluid balance was not positive. However, the rapid fluid infusion may partly explain the rapid progression of pulmonary edema.
The postoperative SICMP diagnostic criteria are: (1) no SICMP symptoms preoperatively (no chest pain and no dyspnea), (2) typical apical ballooning and ventricular wall motion hypokinesia evident on echocardiography, (3) no significant coronary artery obstruction apparent on a coronary angiogram, and (4) rapid elimination of ventricular wall hypomotility [6]. Our case met criteria 1, 2, and 4 but not 3. Preoperatively, she complained of nausea and vomiting but not of SICMP symptoms (criterion 1). In the ICU, basal hypokinesia with severe left ventricle systolic dysfunction (EF=29%) (reverse-type SICMP) was evident on echocardiography (criterion 2) (Fig. 3). The ventricular wall motion abnormality disappeared within 9 days (criterion 4). However (criterion 3), her coronary artery obstruction status was not checked because she refused a coronary angiogram. However, her rapid improvement and mild troponin elevation indicate SICMP rather than an acute coronary syndrome.
We successfully treated a case of near-fatal pulmonary edema attributable to postoperative SICMP in a patient with a recent COVID-19 infection. As the number of infected patients is increasing worldwide, and the number of surgeries on such patients thus also increases, anesthesiologists should be alert to the risk that COVID-19 patients may develop SICMP postoperatively.
Notes
No potential conflict of interest relevant to this article was reported.