Primary Diffuse B-cell Lymphoma of Uterine Cervix: A Case Report
Article information
Abstract
Primary diffuse large B-cell lymphoma (DLBCL) of the female genital tract is a rare case. It is hard to diagnose DLBCL of the uterine cervix before surgery because it is often misdiagnosed as cervical myoma or cervical squamous cell carcinoma. Here, we report a case of cervical DLBCL misdiagnosed as a cervical myoma. A 48-year-old premenopausal woman was referred to the gynecology department due to abnormal uterine bleeding with a normal Papanicolaou smear. The initial diagnosis according to ultrasound and computed tomography was a cervical myoma. She had undergone a hysterectomy with bilateral salpingectomy. The final diagnosis was cervical DLBCL and she was referred to the department of hematology for treatment with Rituximab combined chemotherapy. After six courses of chemotherapy, complete remission was reached. At a 2-year follow-up, the patient is alive without interval change.
INTRODUCTION
Anatomic causes of abnormal uterine bleeding (AUB) in the reproductive age group include polyp, adenomyosis, leiomyoma, and malignancy or hyperplasia [1]. Primary malignant lymphoma of the uterine cervix can cause AUB and is a very rare disease. The incidence is only 0.008% among cervical tumors and 2% among female extranodal lymphomas [2]. The rarity of a cervical diffuse large B-cell lymphoma (DLBCL) makes a timely diagnosis difficult. In this article, we present a case of cervical mass diagnosed as DLBCL and reviewed the associated literatures.
CASE REPORT
A 48-year-old woman, parity 2-0-0-2, was referred to the department of gynecology with AUB in March 2020. She had menorrhagia since last year and had abdominal discomfort, urinary frequency, and dysuria since 1 month ago. Massive vaginal bleeding occurred 6 days ago.
The last normal menstrual period was February 18th, 2020. Her last Papanicolaou (PAP) smear in 2020 was normal. The patient was negative for fever, weight loss, nausea, vomiting, and night sweats. She had a family history of paternal colon cancer and maternal uterine cervix cancer. On the speculum exam, the uterine cervix was without erosion or eversion, and without cervical motion tenderness. A solid mass ranging from uterine lower segment to cervix was noted. It seemed like a cervical myoma inside the cervical internal os.
Initial laboratory data showed anemia (hemoglobin 8.5 g/dL), elevated lactate dehydrogenase (306 U/L), and otherwise normal. Transabdominal ultrasound showed a huge cervical mass. Abdominopelvic computed tomography (CT) (APCT) revealed a 10-cm-sized cervical myoma without evidence of significant lymph node enlargement or distant metastasis in the abdomen and pelvic cavity (Fig. 1A, B). A transabdominal hysterectomy with bilateral salpingectomy was done. DLBCL was diagnosed in the cervix, instead of cervical myoma, according to histopathology (Fig. 2A). Immunohistochemistry of the lymphoma revealed vimentin, CD20, B cell lymphoma 6, MUM1, and Ki-67 positive (Fig. 2B). Desmin, cytokeratin-multi (AE1/AE3), CD3, CD10, CD38, CD138, human herpesvirus-8, caldesmon, and Epstein-Barr virus was negative. Kappa light chain and lambda light chain were not specific.
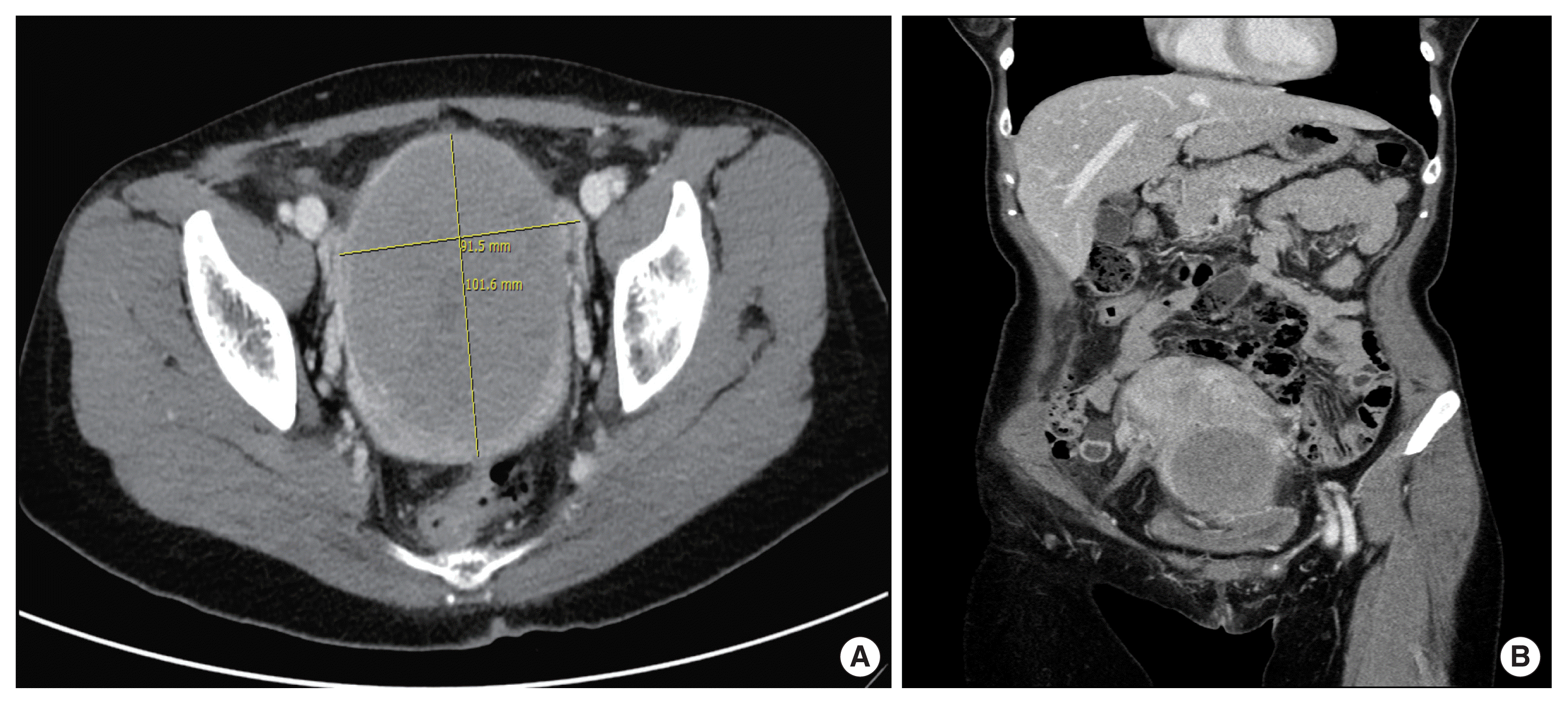
Axial view of computed tomography (CT) scan showed 101.6 mm×91.5 mm sized cervical mass (A). Uterine corpus and the cervical mass are distinguished by the coronal view of CT scan (B).

Pathological evaluation. The tumor is composed of diffuse sheet of large lymphoid cells with abundant cytoplasm, large nuclei, and prominent nucleoli (H&E, ×400) (A). The tumor cells are positive for CD20 (immunohistochemistry for CD20, ×400) (B).
The patient was referred to the department of hematology for further evaluation. For staging work-up, APCT, chest CT, torso positron emission tomography (PET), and bone marrow exam was done. Chest CT was normal. In APCT, compared with the previous CT which was taken only 3 weeks ago, lymph node enlargement along with both common, internal, and external iliac nodal stations and focal nodular enhancement in the deep subcutaneous fat layer of the right lower abdomen newly appeared (Fig. 3A). Also, newly appeared bilateral hydronephrosis due to encasement of distal ureters by lymphadenopathy and pre-vesical mass-like lesion were noted (Fig. 3B, C). In torso PET-CT scan, multiple various degrees of hypermetabolic lesions along the pelvic area, pelvic side wall, anterior abdominal wall, and pre-vesical mass were noted (Fig. 4A, B). Complete obstruction of right ureter was noted (Fig. 4C). In bone marrow exam, no evidence of clonal B-cell population was seen, and leukemia was ruled out. Totally, DLBCL, Ann Arbor stage IIE, IPI L1, was diagnosed and Rituximab-combined immunochemotherapy was done for six cycles every 3 weeks. She has reached complete remission for 2 years until now (Fig. 4D–F). The patient provided written informed consent for the publication for clinical details and images.
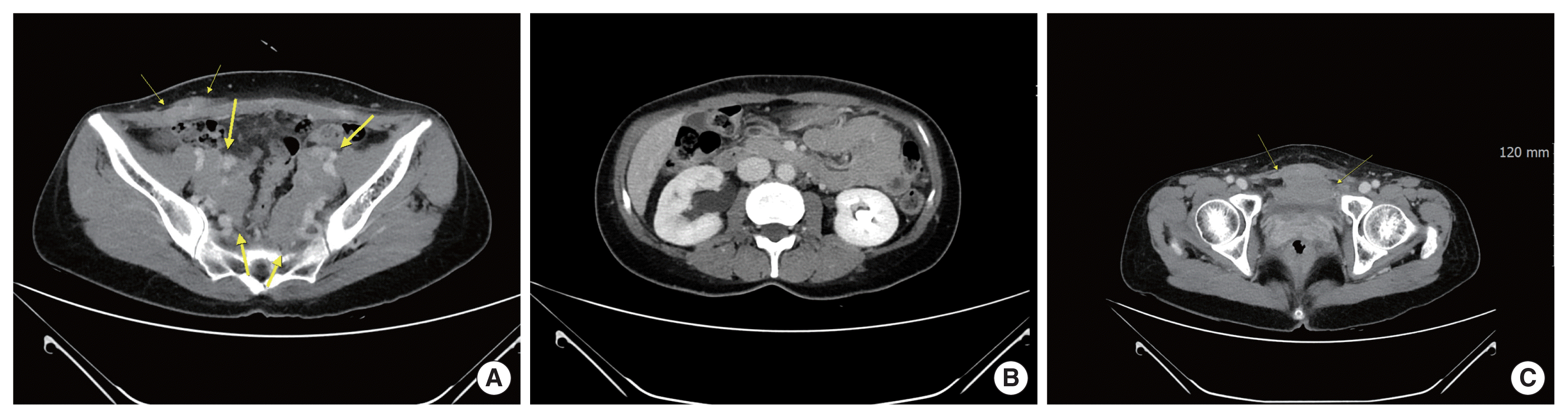
Newly appeared lymph node enlargement along both common, internal, and external iliac nodal stations (thick arrows) and newly appeared focal nodular enhancement in deep subcutaneous fat layer of right lower abdomen (narrow arrows) (A). Newly appeared bilateral hydronephrosis due to encasement of distal ureters by lymphadenopathy, right side more severe obstructive uropathy (B). Newly appeared pre-vesical mass-like lesion (C, arrows).

Multiple variable degree hypermetabolic lesions along pelvic area (SULmax 12.7), pelvic side wall (SULmax 10.3), and anterior abdominal wall (SULmax 8.2) (arrows) (A, B). Hypermetabolic lesions at pre-vesical mass are noted (C). Compared with A–C, all the positron emission tomography–computed tomography scan of the same level shows improvement (D–F).
DISCUSSION
Primary malignant lymphoma of the uterus is a rare disease. Symptoms are non-specific and may include vaginal bleeding (70%), perineal discomfort (40%), and persistent vaginal discharge (20%) [3]. “B” symptoms, such as fever, weight loss, night sweats, and fatigue, are specific to lymphoma [4]; however, may lack in the patients of a cervical DLBCL [2]. To diagnose primary cervical lymphoma, the following criteria must be satisfied: (1) lymphoma is present only at one location (cervix) at the diagnosis; (2) tumor cells are not detected in peripheral blood or bone marrow, so leukemia can be ruled out; and (3) for several months after the initial diagnosis, further lymphoma at remote sites are not found [5]. Because cervical DLBCL is a stromal disease, a PAP smear is not diagnostic [6].
The standard treatment of advanced DLBCL is a combination chemotherapy called rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP): rituximab 375 mg/m2, cyclophosphamide 750 mg/m2, doxorubicin 50 mg/m2, vincristine 1.4 mg/m2 intravenous on the first day of each cycle, and prednisolone 100 mg/day per oral for 5 days of each cycle. This regimen is generally given every 3 weeks for 6 to 8 cycles.
Due to the rarity of cervical primary DLBCL, a standard treatment protocol is not established. Chemotherapy alone, a combination of chemotherapy and radiotherapy, or surgical management have all been reported [2,3,5–10]. Years ago, aggressive treatment of cervical lymphoma such as surgery with radiation therapy was regarded as a mainstay [7]. It is currently changing to a more conservative treatment [8–10]. R-CHOP has proved to be effective and also prevents micrometastasis [9].
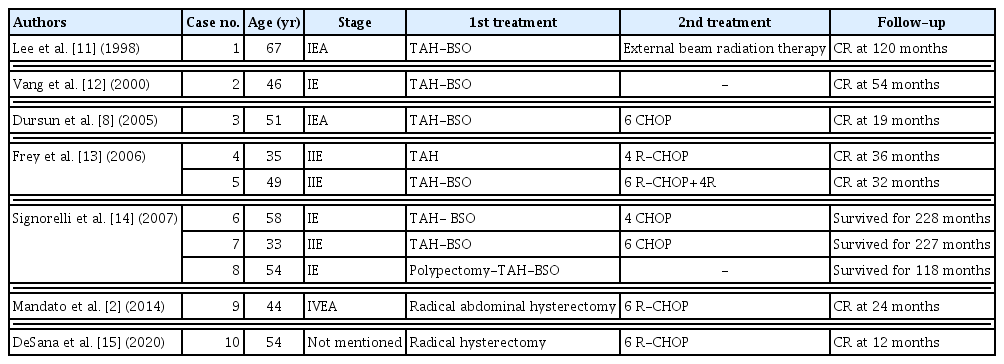
In our case, lymphoma was disseminated just after surgery. We reviewed literatures reporting cases of primary DLBCL of the uterine cervix, treated by surgery (Table 1) [8,11–15]. In Table 1, two cases were treated by surgery alone, seven cases were treated by surgery and immunochemotherapy, and one case was treated by surgery and radiation therapy. Because of the rarity of cervical lymphoma, diagnosis is often delayed and the standard treatment protocol is not established. Multimodal workup and a multi-disciplinary approach with other specialists are required for primary DLBCL of the cervix.
Notes
No potential conflict of interest relevant to this article was reported.