Multiple Markers of Contrast Induced Nephropathy after the Percutaneous Coronary Intervention
Article information
Abstract
Objective
Contrast-induced nephropathy (CIN) frequently occurs after percutaneous intervention. Objective of this study was to investigate the usefulness of serum cystatin C, neutrophil gelatinase-associated lipocalcin (NGAL), urinary kidney injury molecule-1 (KIM-1), and interleukin-18 (IL-18) as early predictors for CIN after percutaneous coronary intervention (PCI).
Methods
In 53 patients who underwent PCI were enrolled. Serum creatinine and cystatin C level were measured immediately before, and 24 hours and 48 hours after catheterization. Serum NGAL, urinary KIM-1, and IL-18 were measured immediately before, and 4 hours, 24 hours, and 48 hours after catheterization. CIN was defined as a rise in creatinine 0.5 mg/dL or 25% above baseline.
Results
CIN occurred in four patients (7.5%). Serum cystatin C levels were higher at 24 hours and 48 hours in CIN patients than in those without CIN (P<0.05). Serum NGAL levels were higher at 48 hours in CIN patients than in those without CIN. Urinary KIM-1 levels were higher at 48 hours in CIN patients than in those without CIN. There were no significant markers of CIN on multi-variate analysis.
Conclusion
In this study, the occurrence of CIN after PCI was 7.5%. Although there were some time-course changes in serum cystatin C and urinary KIM-1 after PCI, there was no significant predictor for CIN after PCI.
INTRODUCTION
Contrast-induced nephropathy (CIN) is responsible for more than 10% of acute renal failure developed in hospitalized patients after diagnostic tests using contrast media. The manifestation of CIN is directly increased as the number of diagnostic tests or procedures using contrast media rises [1–3].
CIN is one of the causes of prolonged hospitalization, increased morbidity, and worsening renal insufficiency leading to chronic renal failure which sometimes requires dialysis [4]. Although the incidence of CIN is less than 1% in normal population, it has been reported in 5.5% of patients who have preexisting renal insufficiency and it is increased up to 50% of patient if they have diabetes [1,2].
Several laboratory markers to predict CIN after coronary angiography have been reported. Cystatin C (CysC) [5–7], neutrophil gelatinase-associated lipocalcin (NGAL) [8–10], urinary kidney injury molecule-1 (KIM-1) [11,12], and interleukin-18 (IL-18) [12–15] have been studied and have showed reliable results.
Also, predictive risk factors for CIN have been well recognized. In a study reporting a risk score for prediction of CIN after percutaneous coronary intervention (PCI), several clinical factors such as hypotension, congestive heart failure, age, anemia, diabetes, amount of contrast media used, estimated glomerular filtration rate (GFR), and use of intra-aortic balloon pump have been reported [16].
The purpose of this study was to identify the prevalence and clinical features of CIN among patients underwent PCI and evaluate the usefulness of the serum CysC, serum NGAL, urinary KIM-1, and urinary IL-18 as the early predictors for CIN by measuring of each marker serially.
MATERIALS AND METHODS
A total of 53 patients who underwent PCI for stable angina at Soonchunhyang University Seoul Hospital were enrolled from May 2009 to November 2009. This study was approved by the Institutional Review Board of Soonchunhyang University Seoul Hospital (IRB approval no., 2009-042). Each participant provided written informed consent. Exclusion criteria were as follows: (1) serum creatinine level (Cr) more than 2.0 mg/dL; (2) GFR less than 30 mL/min/1.73 m2 at stable condition; (3) chronic renal failure patients who is on hemodialysis; (4) multiple myeloma; (5) pulmonary edema; (6) uncontrolled hypertension (systolic blood pressure [BP] more than 160 mm Hg or diastolic BP more than 100 mm Hg); (7) a history using contrast media within 2 days; (8) allergic reaction to contrast media; (9) pregnancy; and (10) patients who were taking theophylline, dopamine, mannitol, fenoldopam, and N-acetylcysteine.
CIN was defined as increase of serum Cr 0.5 mg/dL or 25% above baseline within 48 hours after using contrast media [17]. GFR was calculated by modification of diet in renal disease equation [18]. Hypertension was defined as systolic BP more than 140 mm Hg and/or diastolic BP more than 90 mm Hg at resting and/or treatment with antihypertensive medication. Diabetes was also defined as fasting blood glucose more than 126 mg/dL and/or treatment with oral hypoglycemic agents. Left ventricular systolic function was determined through transthoracic echocardiography by measurement of left ventricular ejection fraction.
Coronary angiography was performed using standard techniques and interventional instruments were decided by operator’s discretion. Non-ionic low osmolar contrast media was used regardless of the company of product. Intravenous 0.9% isotonic saline, as an infusion rate of 1 mL/kg/hr, was given before, and after 12 hours of procedure for all patients.
We performed laboratory test including lipid profile, pro-brain natriuretic peptide, and C-reactive protein before PCI. Serum Cr was measured immediately before and, 4, 24, and 48 hours after PCI. The serum CysC was measured by ELISA kit (Dade Behring, Deerfield, IL, USA) and serum NGAL by ELISA kit (BioPorto, Gentofte, Denmark) immediately before, 4, 24, and 48 hours after PCI, which were stored in −70°C refrigerator after centrifugation. Fresh urine samples collected early in the morning and stored at −70°C refrigerator were centrifuged to remove cellular components. Urinary KIM-1 and IL-18 were measured just before, 4, 24, and 48 hours after PCI and analyzed by ELISA kit (R&D Systems, Minneapolis, MN, USA). Urinary KIM-1 and IL-18 were adjusted with urinary Cr in the early morning spot urine.
Data are presented as numbers and frequencies for categorical variables and as mean±standard deviation for continuous variables. For comparisons between the patients with and without CIN, independent sample t-test for continuous variables and chi-square test was used for categorical variables. The changes of each marker were analyzed by repeated measures analysis of variance with Bonferroni test. Correlations between continuous variables were analyzed by the Pearson’s correlation. To identify the independent predictive factors for CIN, univariate and multivariate logistic regression was used. All statistical analyses were conducted with the SPSS ver. 13.0 (SPSS, Chicago, IL, USA) and two-sided P-values <0.05 were considered statistically significant.
RESULTS
1. Patient characteristics
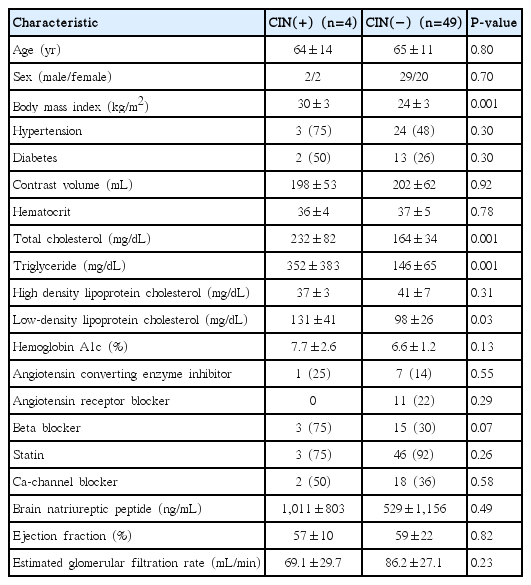
A total of 53 patients, CIN occurred in four patients (7.5%). Baseline characteristics were shown at Table 1. There were no significant differences in age, sex, hypertension, and diabetes between patients with CIN (CIN group) and without CIN (non-CIN group). Also, contrast volume in CIN group was not different from non-CIN groups (198 mL vs. 202 mL, P=0.92). However, body mass index (BMI) in CIN group was significantly higher than non-CIN group (30±3 kg/m2 vs. 24±3 kg/m2, P=0.001)
There were no significant differences between the groups on laboratory findings and drug history except lipid profile. Total cholesterol, triglyceride, and low-density lipoprotein (LDL) cholesterol in CIN group were significantly higher than non-CIN group (232±82 mg/dL vs. 164±34 mg/dL, P=0.001; 352±383 mg/dL vs. 146±65 mg/dL, P=0.001; 131±41 mg/dL vs. 98±26 mg/dL, P=0.03, respectively).
2. Glomerular filtration rate, serum creatinine, and cystatin C level
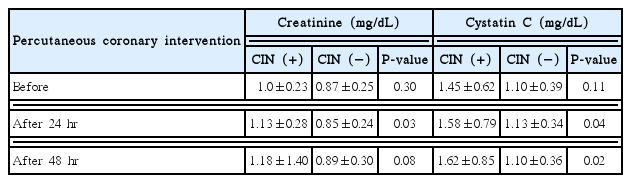
The baseline GFR and serum Cr showed no difference between two groups. However, the serum Cr, measured at 24 hours after PCI, was significantly increased in CIN group than non-CIN group (1.13±0.34 mg/dL vs. 0.85±0.24 mg/dL, P<0.05). The serum Cr, measured after at 48 hours after PCI, was not different between two groups.
In case of serum CysC level, there was no significant difference at baseline between two groups. However, the serum CysC, measured at 24 and 48 hours after PCI, were significantly higher in CIN group than non-CIN group (1.58±0.79 mg/dL vs. 1.13±0.34 mg/dL, P=0.04; 1.62±0.85 mg/dL vs. 1.10±0.36 mg/dL, P=0.02, respectively) (Table 2).
3. The serum neutrophil gelatinase-associated lipocalcin, urinary kidney injury molecule-1, and urinary interleukin-18
The serial alteration of the serum NGAL, urinary KIM-1, and urinary IL-18 were shown in Table 3. The baseline serum NGAL level in CIN group was not different from non-CIN group. However, serum NGAL in non-CIN group was significantly increased at 24 hours after PCI, as compared with baseline (161.3±113.3 ng/mL vs. 105.0±73.3 ng/mL, P<0.05). However, in CIN group, there was no significant difference (Fig. 1).

Time-course changes in serum NGAL. NGAL, neutrophil-gelatinase-associated lipocalcin; CIN, contrast induced nephropathy. *P<0.05 (vs. baseline).
The urinary KIM-1 at 24 hours after PCI did not show difference between two groups. But, urinary KIM-1 at 48 hours after PCI was significantly higher in the CIN group than non-CIN group (1.4±0.6 ng/mL vs. 0.7±0.5 ng/mL, P<0.05). The urinary KIM-1 in at 4 hours after PCI in non-CIN group was significantly decreased, compared with at baseline (0.3±0.3 ng/mL vs. 0.5±0.4 ng/mL, P<0.05). The urinary KIM-1 were increased at 24 and 48 hours after PCI (0.8±0.5 ng/mL vs. 0.3±0.3 ng/mL, 0.7±0.5 ng/mL vs. 0.3±0.3 ng/mL, respectively; P<0.05). In contrast, time-course changes were not found in CIN group (Fig. 2).
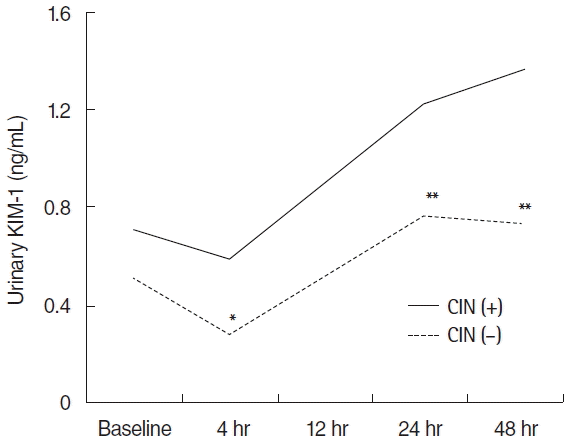
Time-course changes in urinary KIM-1. KIM-1, kidney injury molecule-1; CIN, contrast induced nephropathy. *P<0.05 (vs. baseline). **P<0.05 (vs. after 4 hr).
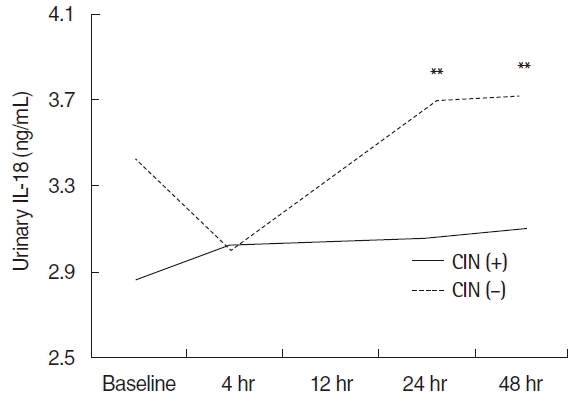
The urinary IL-18 at 24 and 48 hours after PCI was significantly higher in the non-CIN group compared with at 4 hours (Fig. 3). However, time-course changes were not found in CIN group.
4. Predictive factors for contrast-induced nephropathy by univariate and multivariate logistic regression analysis
In univariate logistic regression analysis, BMI (odds ratio [OR], 1.7; P=0.013), total cholesterol (OR, 1.03; P=0.035), 48 hours CysC (OR, 8.22; P=0.041), and 4 hours urinary KIM-1 (OR, 9.4; P=0.04) were significant variables for CIN (Table 4). However, there was no significant predictor for CIN in multivariate analysis.
DISCUSSION
The major findings of the present analyses are as follows. (1) Among 53 patients who underwent PCI, four patients (7.5%) were developed the CIN. (2) Baseline BMI, total cholesterol, LDL-cholesterol, and triglyceride in CIN-group were higher than non-CIN group. (3) Serum CysC levels at 24 hours and 48 hours after PCI were higher in CIN group. (4) Serum NGAL was increased in 4 hours after PCI and reached to peak level at 24 hours PCI and begun to decrease at 48 hours after PCI. (5) Urinary KIM-1 and urinary IL-18 were temporally decreased 4 hours after PCI and then reached to peak level at 24 hours and begun to decrease at 48 hours after PCI, especially in CIN-group.
1. Prevalence and clinical features of contrast-induced nephropathy
The intravascular injection of contrast media is a common cause of CIN. It is well known that CIN is associated with prolonged hospitalization, increased mortality, and prolonged renal injury after coronary angiography or repeated PCI [4]. The development of CIN is low in patients with normal renal function, varying from 0% to 10% [19]. However, its incidence is increased to 20% in case of patients whose baseline serum Cr was between 1.5 and 2.0 mg/dL [12].
2. Usefulness of cystatin C and neutrophil gelatinase-associated lipocalcin in contrast-induced nephropathy
In chronic kidney disease patients, it needs to try to prevent development of CIN. But, it is difficult to assess the risk of CIN only by serum Cr. Although serum Cr is a standard test for an acute renal injury, it is not accurately correlated with acute renal dysfunction. Especially, in acute renal injury, serum Cr was not elevated for several days after beginning of renal tubular damage [13]. Though Coca et al. [20] reported about 21 serum and urine makers for diagnosis of acute renal injury by systematically review of 31 studies related with acute renal injury, more investigations are needed to evaluate their effectiveness. Fortunately, many studies are conducted regarding to serum CysC, urinary NGAL, IL-18, KIM-1, and L-type fatty acid binding protein, recently [14,15].
Serum CysC is one of the promising surrogate markers for GFR and it could be superior to serum Cr. It is useful in old ages because it is well correlated with renal dysfunction regardless of age and not affected by sex, and muscle mass [16,17]. Kimmel et al. [21] also reported that CysC is a more reliable marker than serum Cr for prediction of CIN in 54 patients who underwent coronary angiography.
NGAL is known to be a predicted marker for acute ischemic renal injury. Mishra et al. [22] studied 20 patients who developed acute renal injury among 71 patients who received cardiopulmonary bypass for congenital heart disease surgery. According to the study, acute renal injury was affected by serum and urinary NGAL at 2 hours after surgery, and operation time and urinary NGAL was most reliable independent maker for acute renal injury in univariate analysis. However, in our study, there was no relation between NGAL and acute renal injury. It might be explained by limited numbers of CIN patients in our study. Although CIN is a serious complication after coronary angiography and PCI, it is difficult to assess acute renal injury only with serum Cr or Cr clearance because patients are routinely discharged in 48 hours.
In a study, conducted to angina pectoris patients who had normal Cr after coronary angiography and PCI, serum and urinary CysC and NGAL were checked before, 2, 4, 8, 24, and 48 hours after intervention [23]. Among them, CIN was occurred in 11%, and serum and urinary NGAL were highest at 2, 4 hours after PCI. In contrast, CysC in CIN patients was slightly higher at 8, 24 hours after PCI.
It has previously reported that NGAL is highly sensitive and it can function as an early prognostic marker for acute renal injury after PCI. Elevated NGAL caused by inflammatory reaction of neutrophil after PCI. NGAL is increased after PCI because it is increased in atheromatous plaque [24]. NGAL showed the maximum elevation after 24 hours in non-CIN group. However CysC and serum Cr were not elevated in this study.
3. Usefulness of kidney injury molecule-1 in cystatin C
KIM-1 is a transmembrane tubular protein which increases dramatically after injury in proximal tubule epithelial cells during ischemic and toxic renal injury [25]. It has been used as a new marker for diagnosis of acute renal injury [26]. It has been reported that urinary KIM-1 might serve as a useful biomarker for renal proximal tubular injury facilitating the early diagnosis of the disease because it was significantly increased in patients with acute ischemic tubular necrosis compared with chronic renal failure or other acute renal injury [11]. In this study, KIM-1 was significantly increased at 24 and 48 hours after contrast injection in non-CIN group and 48 hours KIM-1 was higher in CIN group compared with non-CIN group. Yet, it needs larger studies in order to confirm KIM-1 as a prognostic marker for renal injury because it only showed significant difference in non-CIN group.
4. Usefulness of interleukin-18 in cystatin C
In a study conducted with 13 CIN group and 27 non-CIN group after coronary angiography, urinary IL-18 was significantly increased in the CIN group and independent predictive marker for later major cardiac events [13]. However, another study with 15 CIN and 36 non-CIN group after PCI, urinary IL-18 was not significantly different [14]. In our study, IL-18 was significantly increased at 24 and 48 hours after PCI compared with 4 hours after PCI. But it needs more studies to confirm that IL-18 is affected by renal injury actually, because IL-18 was increased 24 and 48 hours after PCI even in non-CIN group. Since most studies regarding IL-18 with renal injury were relatively small-sized studies, it needs larger studies to identify urinary IL-18 as an early predictor for CIN.
5. Limitations
The limitations of this study are as follows. First, the number of patients, especially CIN patients was relatively small. Second, though patients with normal renal function were studied, variable distribution of GFR by multiple factors was related with analysis of result. Later on, it needs to identify in terms of influence of renal dysfunction with large numbers of patient who is divided group by GFR. Third, it was often difficult to verify consistency about results from process of samples which were affected by many factors, such as obtaining samples, centrifuge, and cold storage.
6. Conclusion
In this study, the occurrence of CIN after PCI was 7.5%. Although there were some time-course changes in serum CysC and urinary KIM-1 after PCI, there was no significant predictor for CIN after PCI.