Obstructive Jaundice Caused by Tuberculous Lymphadenopathies
Article information
Abstract
Although pulmonary tuberculosis is known to be the most common type in tuberculosis, it actually can affect any organ system. However, abdominal type is very rare among the extra-pulmonary types, and obstructive jaundice caused by lymphadenopathies due to tuberculosis is especially uncommon manifestation even in endemic areas. Tuberculous lymphadenopathies can mimic lymphadenopathies by other metastatic tumors or lymphoma, thus early correct diagnosis is very important for avoiding unnecessary surgical interventions. Here, we reported two cases of obstructive jaundice caused by tuberculous lymphadenopathies. Both were treated with anti-tuberculosis medications and endoscopic retrograde biliary drainage without surgery.
INTRODUCTION
Abdominal tuberculosis (TB) is known to be 12–25% of all extra-pulmonary TB [1], and diagnosis of lymphadenopathies (LAPs) by abdominal TB is not simple, because those often mimic LAPs by malignant tumor or lymphoma. For this reason, surgical interventions have been carried out globally for differentiating benign lesions from malignancies up to date [2]. However, if correct diagnosis can be established without surgical interventions, it would be helpful for saving patients’ time and cost as well as avoiding severe complications from invasive procedures. Here, we report two cases of obstructive jaundice due to pericholedochal LAPs by TB, which were treated with anti-TB medications and endoscopic retrograde biliary drainage (ERBD) without surgery.
CASE REPORTS
1. Case 1
A 29-year-old man visited Eulji University Hospital for intermittent epigastric pain. Seven months ago, he was diagnosed with TB abscess on his right axilla and had taken anti-TB medications for six months.
Physical examinations revealed jaundice and right upper abdominal tenderness, but there was no fever. Laboratory data included the followings: hemoglobin 14.9 g/dL (normal range, 12 to 16 g/dL); white blood cell 9,280/μL (normal range, 3,800 to 10,500/μL); C-reactive protein 1.28 mg/dL (normal range, 0 to 0.5 mg/dL); aspartate aminotransferase (AST) 188 IU/L (normal range, 1 to 34 IU/L); alanine aminotransferase (ALT) 151 IU/L (normal range, 1 to 34 IU/L); alkaline phosphatase (ALP) 178 IU/L (normal range, 25 to 100 IU/L); γ-glutamyl transpeptidase (γ-GTP) 450 IU/L (normal range, 1 to 35 IU/L); and total bilirubin 2.9 mg/dL (normal range, 0.1 to 1.2 mg/dL). Acid-fast bacillus (AFB) stain, culture, and polymerase chain reaction (PCR) for Mycobacterium tuberculosis of sputum were negative.
Abdominal computed tomography (CT) revealed narrowed mid common bile duct (CBD) with dilated intrahepatic bile duct and proximal CBD, in addition to suspicious fistula tract between lymph node and mid CBD (Fig. 1A). Several central necrotic LAPs at portocarval and porta hepatis area were also observed. Endoscopic retrograde cholangiopancreatography (ERCP) demonstrated narrowed mid CBD with dilated intrahepatic bile duct and proximal CBD, but fistula tract was not observed on the cholangiogram. Bile juice was aspirated for culture and PCR for M. tuberculosis and other mycobacteria (MTB-PCR), and finally, ERBD was inserted in the CBD. All bile examinations yielded negative results.

(A) Abdominal CT at admission revealed mild bile duct dilatation due to lymphadenopathies (arrowhead) and suspicious fistula tract between lymph node and CBD (arrow). (B) After five weeks with anti-tuberculosis medications and endoscopic retrograde biliary drainage, abdominal CT showed regressed previous enlarged lymph nodes and improvement of dilated CBD. CT, computed tomography; CBD, common bile duct.
When considering his tuberculous history and CT findings, obstructive jaundice due to pericholedochal LAPs by TB was strongly suspected. His abdominal pain and jaundice was improved gradually with anti-TB medications and ERBD. Five weeks later, followed abdominal CT demonstrated regressed previous multiple enlarged lymph nodes and improvement of dilated CBD (Fig. 1B). Laboratory data was also within normal limit. ERBD was removed after three months from initial insertion.
2. Case 2
A 29-year-old man visited Eulji University Hospital with itching sensation and icteric sclera. He had no history of specific disease. At physical examinations, he had jaundice with right upper abdominal tenderness.
Laboratory data revealed the followings: hemoglobin 13.7 g/dL (normal range, 12 to 16 g/dL); white blood cell 8,270/μL (normal range, 3,800 to 10,500/μL); C-reactive protein 0.2 mg/dL (normal range, 0 to 0.5 mg/dL); AST 49 IU/L (normal range, 1 to 34 IU/L); ALT 83 IU/L (normal range, 1 to 34 IU/L); ALP 285 IU/L (normal range, 25 to 100 IU/L); γ-GTP 63 IU/L (normal range, 1 to 35 IU/L); and total bilirubin 6.82 mg/dL (normal range, 0.1 to 1.2 mg/dL). Tumor marker, carbohydrate antigen 19-9, was within normal limit.
Abdominal CT demonstrated compression of mid CBD with dilated proximal CBD and intrahepatic bile duct due to extrinsic compression of portocarval and porta hepatis LAPs (Fig. 2A). The enlarged portocarval lymph node had a hypo-attenuated lesion in the center with surrounding enhanced rim. Duodenoscopy revealed several whitish plaques and a fistula opening near the ampulla of Vater (Fig. 3A). Cholangiogram revealed stricture of mid CBD with dilated intrahepatic bile duct (Fig. 3B). Biopsy with AFB stain and MTB-PCR was performed on the fistula opening for a doubt of TB, and the result showed ulcer with epithelioid cell granuloma (Fig. 4). Another biopsy for TB was performed in the CBD, but it showed chronic inflammation with negative result of AFB stain and PCR. Finally, ERBD was inserted in the CBD for decompression of dilated bile duct. In addition, interferon gamma (IFN-γ) release assay (QuantiFERON; QIAGEN company, Duesseldorf, Germany), which was performed at the day of ERCP, represented positive result. We assumed the most possible diagnosis to obstructive jaundice due to pericholedochal LAPs by TB with fistula opening near the ampulla of Vater. Therefore, anti-TB medications were started.

(A) The dilated proximal CBD (arrowhead) due to extrinsic compression of portocarval and porta hepatis lymphadenopathies (arrow) was noted on computed tomography scan. (B) The previous dilated proximal CBD and intrahepatic bile duct (arrowhead) with enlarged lymph nodes (arrow) were regressed. CBD, common bile duct.
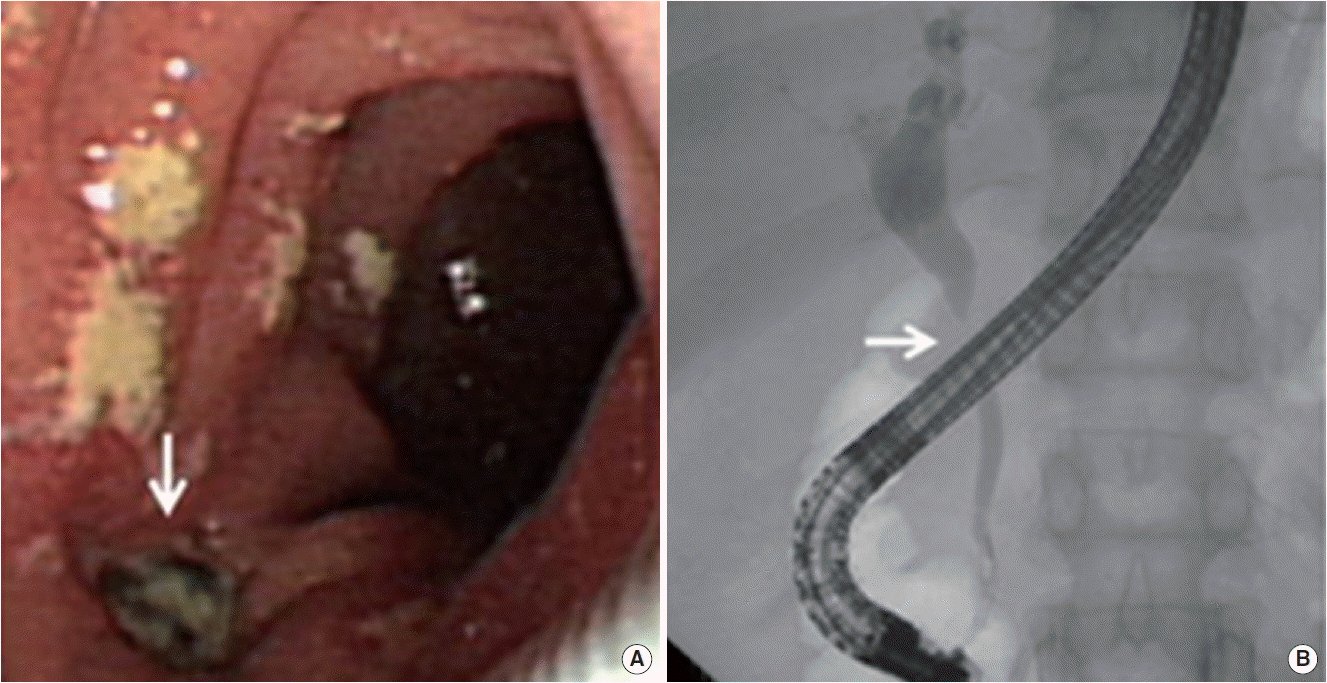
(A) Duodenoscopy revealed suspicious fistula opening (arrow). (B) Cholangiogram revealed stricture of mid common bile duct with dilated intrahepatic bile duct (arrow).
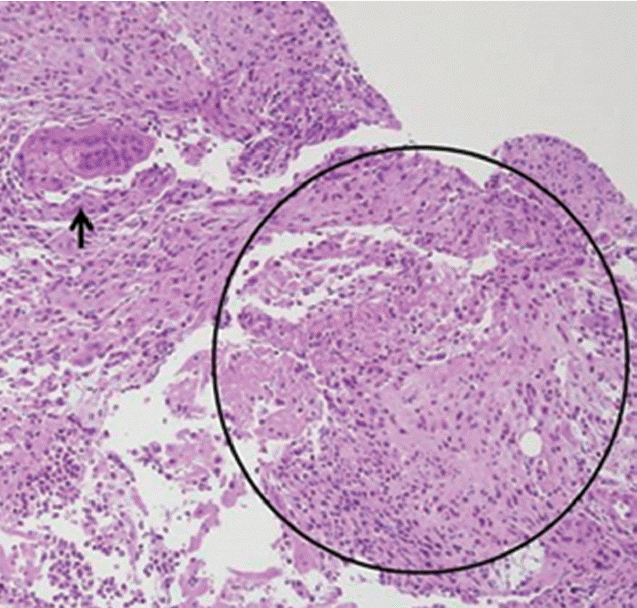
The biopsy result on the fistula opening showed ulcer with epithelioid cell granuloma (circle) and Langhans giant cell (arrow) (H&E, ×100).
Eight months later, esophagogastroduodenoscopy noted that previous fistula was resolved with scar change. In abdominal CT, the previous delated proximal CBD and intrahepatic bile duct with enlarged lymph nodes were also regressed (Fig. 2B.). There was no recurrence of symptom during the follow-up period, thus ERBD was removed.
DISCUSSION
Obstructive jaundice secondary to abdominal TB is rare, even in TB endemic areas. It can be caused by compression of enlarged tuberculous lymph nodes, enlarged tuberculous head of pancreas or tuberculous mass of the retroperitoneum, in addition to the stricture of biliary tree as a result of tuberculous infection [2]. Several cases of them can mimic malignancies radiologically, and these may lead to unnecessary surgical interventions [3]. Therefore, early correct diagnosis is crucial because, in most cases, the disease can be successfully treated conservatively.
Diagnosis of LAPs by TB is not easy, and gold standard for diagnosis has not been founded yet. Positive personal or familial history of TB can be useful for the diagnosis, but it appears in less than 30% of patients [4]. Laboratory findings are non-specific; anemia, lymphocytopenia or pancytopenia, and elevated transaminase levels are most commonly encountered [5].
Although LAPs by TB can be suspected when low-density center with surrounding enhanced rim is detected on CT scan, CT cannot differentiate LAPs by TB from metastatic malignancies or lymphoma [3]. Thus, fine needle aspiration (FNA) biopsy guided with ultrasonography or CT may be helpful for diagnosis, and the caseous necrosis and Langhans giant cell in granuloma is said to be the typical histologic finding of TB [6]. However, FNA biopsy has some worries about dissemination of M. tuberculosis, even cancer cells before the correct diagnosis is made. Moreover, some authors consider that it is not reliable because of high rate of false-negative result [1].
ERCP helps to confirm the location and the degree of biliary stricture, to decompress biliary tract using stent for relieving jaundice and to obtain bile juice or tissue for critical exams such as culture, AFB stain and TB-PCR [3]. However, if the biliary obstruction is due to extrinsic compression of lymph nodes, and it doesn’t have any invasion of bile duct wall itself or affected lesions such as fistula, clinicians may not be able to expect appropriate results from the bile exams [7]. In the first case of this report, fistula tract between lymph node and CBD was suspicious on CT scan, but there was no definite fistula opening in duodenoscopy. Besides, all examinations from the aspirated bile juice for culture and MTB-PCR also showed negative results. Therefore, diagnosis by only ERCP may not be sufficient in the cases of secondary obstructive jaundice due to extrinsic compression of LAPs by TB.
Recently, IFN-γ release assays (IGRAs, QuantiFERON) is spotlighted for the diagnosis of latent TB. It is more specific to M. tuberculosis than tuberculin skin test, and is not affected by prior BCG vaccination. In addition, it does not cross-react with nontuberculous mycobacteria [8]. In the second case of this report, although the patient had no history of TB in his life, we could suspect the possibility of LAPs by TB with positive result of IGRAs.
While anti-TB medication without surgical interventions is mostly desirable, and our two patients were improved with anti-TB medications and biliary stent only, there are some practical limitations in conservative management alone. First, multi-drug resistant M. tuberculosis is in a growing trend. Second, recurrent inflammation of biliary duct can cause irreversible scar change with progressive stenosis of the bile duct [6]. Actually, from 1976 to 2014, sixteen cases of pericholedochal LAPs by TB which induced obstructive jaundice were reported officially in Korea except this report. Five cases were treated by anti-TB medications alone or anti-TB medications with ERBD, but the other eleven needed surgical interventions accompanied by anti-TB medications [2]. Additionally, choledochoduodenal fistula as a complication of TB was closed with anti-TB medications only in few cases [9]. Thus, a proper combination of methods for diagnosis and treatment should be further emphasized.
In conclusion, considering the high prevalence of TB in Korea, pericholedochal LAPs by TB is likely to be encountered often in the field. Therefore, clinicians need to consider the possibility of LAPs by TB in the patients who have any TB-associated risk factor such as history of TB or residency in endemic areas.