Benign Prostatic Hyperplasia Mimicking a Symptomatic Rectal Submucosal Tumor
Article information
Abstract
We report the case of a 56-year-old man with a prostatic mass that extended into the rectal mucosa. He experienced constipation and anal bleeding for 6 months. He underwent surgical ablation for an approximately 5-cm, benign, subepithelial cystic mass in the rectum, which was adjacent to a 5-cm solid mass located on the prostatic gland seen on computed tomography and magnetic resonance imaging (MRI) of the pelvis. One year after the surgery, the patient had recurrent anal bleeding with difficulty defecating. The pelvic MRI scan showed a solid mass with heterogeneous enhancement that was compressing the rectum. The sigmoidoscopic exam showed a 4-cm mass protruding through the anterior rectal mucosa 7-cm above the anal verge. Ultra-low anterior resection with ileostomy and prostatectomy was performed for curative resection of the mass with extension into the rectum. However, the pathologic report showed massive benign prostatic hyperplasia involving the rectum, but not penetrating into the rectal mucosa. The patient did not complain of any symptoms including constipation and anal bleeding, until 18 months after the surgical resection. This is the first reported case of benign prostatic hyperplasia mimicking a rectal submucosal tumor in a patient presenting with anal bleeding and constipation.
INTRODUCTION
Benign prostatic hyperplasia (BPH) is a common disease in men, and the prevalence of BPH increases with age [1]. BPH presents with obstructive and irritating lower urinary symptoms including weak urinary stream, hesitancy, incomplete voiding, nocturia, urgency, and frequency [1]. In contrast, gastrointestinal manifestations due to extrinsic compression of the anterior rectum are very rare in patients with BPH. Here, we report the case of a 56-year-old man who presented with anal bleeding and constipation because of a benign prostatic mass mimicking a rectal submucosal tumor (SMT).
CASE REPORT
A 56-year-old man was referred to Seoul National University Hospital for treatment of a rectal subepithelial mass. He had experienced new onset constipation and anal bleeding for the past 6 months. Outside our hospital, he previously underwent a colonoscopic exam, prostate magnetic resonance imaging (MRI), and a computed tomography (CT) scan of the abdomen and pelvis to exclude colorectal cancer. On the colonoscopic exam, an enteric cyst with pus was found at the rectum. Although he received empiric antibiotic therapy to treat a suspected infected enteric cyst at the rectum, his symptoms of constipation and anal bleeding had worsened. He had been diagnosed with BPH and 5 years previously underwent potassium-titanyl-phosphate laser vaporization of the prostate to relieve his obstructive lower urinary symptoms. He had not experienced any complications immediately after the surgical intervention. Otherwise, his past medical history was unremarkable.
Physical examination revealed no abnormality except a palpable mass in the anterior rectum on digital rectal examination. Laboratory studies showed his serum prostate-specific antigen (PSA) level was 39.80 ng/mL (range, 0 to 3 ng/mL), white blood cell count was 4,320/mm3, hemoglobin level was 15.1 g/dL, and platelet count was 193,000/mm3. Both his serum C-reactive protein and chemistry panel were within normal ranges. The abdominal and pelvic CT and prostate MRI scan performed at an outside hospital showed a 5.3-cm, well-defined, multiseptated cystic mass in the anterior aspect of the rectum, which was adjacent to a 5.0-cm, well-defined, heterogeneous, solid mass with enhancement in the retrovesical space (Fig. 1A, B). A repeat colonoscopy was performed to determine the characteristics of the rectal subepithelial mass. On the endoscopic exam, the lumen of the rectum was almost obstructed by an approximately 5-cm rectal subepithelial cystic mass, with a hyperemic mucosal surface about 5 cm above the anal verge (Fig. 2A). On the transrectal ultrasound (TRUS), a 5-cm, solid, exophytic mass with a 6-cm, fluid-filled cystic cavity was seen originating from the prostate gland. TRUS-guided fine needle aspiration and biopsy (FNA/B) was performed to evaluate both the cystic fluid and the solid mass, respectively. The cystic fluid was dark, bloody, and included some macrophages and many lymphocytes with degenerative blood on the histopathologic examination. In addition, there were no tumor cells in the biopsy specimen from prostatic mass.

Radiologic findings. (A) The initial CT scan of the abdomen and pelvis and (B) a prostate MRI show a well-defined, multiseptated, cystic mass in the anterior aspect of the rectum (white arrow) and a well-defined heterogeneous solid mass with enhancement in the retrovesical space (black arrow). (C) The follow-up CT scan of the abdomen and pelvis and (D) a prostate MRI 6 months after surgical ablation of the cystic mass show no interval change in the size of the solid mass in the retrovesical space (black arrow). (E) The follow-up CT scan of the abdomen and pelvis and (F) a prostate MRI about 12 months after surgical ablation of the cystic mass show no interval change in the size of the solid mass in the retrovesical space (black arrow). CT, computed tomography; MRI, magnetic resonance imaging.
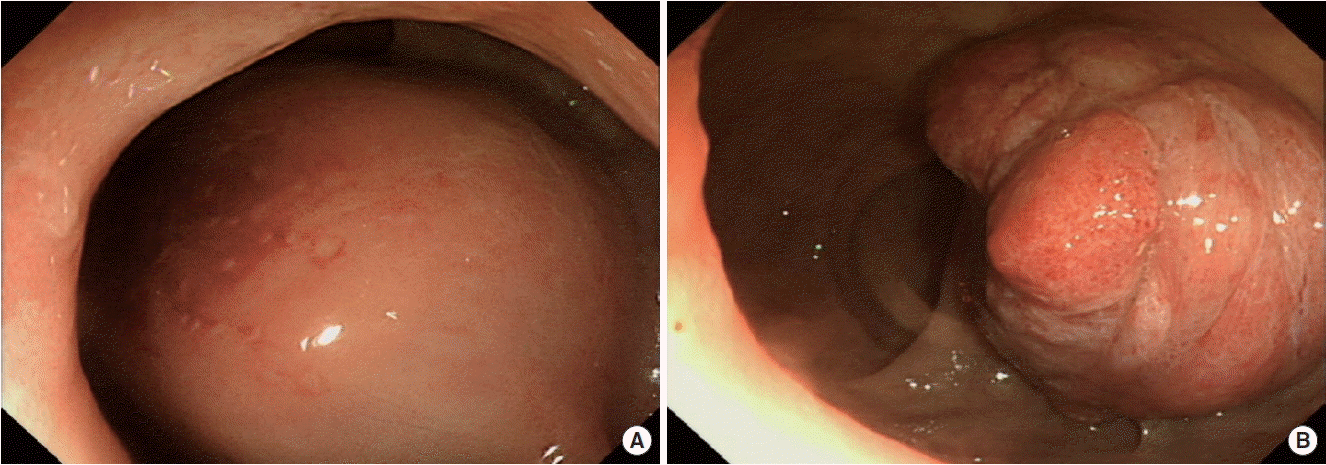
Sigmoidoscopic findings. (A) The initial endoscopic exam showed a 5-cm rectal subepithelial cystic mass with a hyperemic mucosal surface. (B) The follow-up endoscopic exam showed a 4-cm, protruding mass 7 cm above the anal verge.
The patient received surgical therapy for the rectal subepithelial cystic mass, which was unresponsive to empiric antibiotics. On the operative findings, the cystic mass was regarded as a benign inflammatory cyst like the preoperative TRUS findings and was removed by surgical ablation. The pathologic examination showed a cyst lined with a fibrotic capsule without an epithelial lining on the cystic wall (Fig. 3A). These findings suggest that the rectal subepithelial cystic mass was a benign inflammatory cyst adjacent to the rectal wall, which was caused by prostatitis in the background of BPH. Thus, ciprofloxacin was administered for 3 weeks to resolve the remaining prostatitis.

Pathologic findings. (A) Hematoxylin and eosin (H&E) staining of the cystectomy specimen showed a uniseptal cyst, which was lined by a fibrotic capsule without an epithelial lining on the cystic wall (H&E, scan view, ×1). (B) A gross view of the resected specimen. A 5.0-cm, well-defined, heterogeneous, solid rectal mass (black arrow) is shown from intraluminal side. (C) H&E staining of the resected rectal mass specimen showed benign prostatic tissue and rectal tissue (H&E, ×40). (D) Immunohistochemical staining for prostate-specific antigen (PSA) in the rectal mass specimen (PSA stain, ×40).
Six months after the surgical ablation, the enlarged prostate was still palpable on digital rectal examination. The patient’s serum PSA level was 22.89 ng/mL. However, he did not have any symptom of obstructed defecation. On a CT scan of the abdomen and pelvis and a prostate MRI, there was also no interval change in the size of the heterogeneous prostatic mass in the retrovesical space compared to the radiologic images before the surgery (Fig. 1C, D).
One year after the surgery, however, he experienced relapsed symptoms of difficulty in defecation with anal bleeding. He underwent a follow-up CT scan of the abdomen and pelvis, a prostate MRI, and sigmoidoscopy to evaluate the recurrence of the rectal subepithelial cystic mass. On the radiologic findings, there was no significant interval change in the size of the mass (Fig. 1E, F). In contrast, the sigmoidoscopic exam showed a 4-cm soft mass protruding through the anterior rectal mucosa 5 cm above the anal verge (Fig. 2B). On the pathologic findings from the rectal mucosa by endoscopic biopsy, chronic active inflammation with pseudoepitheliomatous hyperplasia of the squamous epithelium was detected. Ultra-low anterior resection with ileostomy and prostatectomy was performed for curative resection of the mass with extension into the rectum to relieve the obstructive symptoms and exclude a malignant tumor (Fig. 3B). The pathologic reports showed massive BPH not penetrating into but involving the rectum (Fig. 3C, D).
Eighteen months after the second surgery, the patient did not complain of any symptoms including obstructive defecation and anal bleeding. His serum PSA level also decreased to 0.18 ng/mL.
DISCUSSION
SMT refers to a mass or mass-like lesion that protrudes into the lumen of the gastrointestinal tract and is covered with intact mucosa. Extramural SMT is most frequently caused by direct invasion of perirectal tumors or peritoneal carcinomatosis [2]. Prostate cancer is a differential diagnosis of extramural rectal SMTs. Gastrointestinal manifestation of prostate cancer is an obstructing rectal mass and/or gastrointestinal bleeding [3,4], which develops by direct invasion or metastasis in 1.5% to 11% of patients with prostate cancer [5]. In contrast, benign rectal SMTs are mostly asymptomatic and are usually found incidentally during routine endoscopy [6]. In our case, a rectal subepithelial mass was detected due to the symptoms of obstructive defecation and bleeding in a patient with BPH, not prostate cancer. To the best of our knowledge, this is the first case of a benign prostatic mass mimicking a symptomatic rectal SMT.
In this case, the rectal purulent cystic subepithelial mass was detected on the endoscopic exam in a patient presenting with constipation and anal bleeding. The symptomatic rectal subepithelial cyst was adjacent to a solid prostatic mass and was lined with a fibrotic capsule without an epithelial lining. It suggests that the benign inflammatory cyst originated from the prostate gland not the rectal wall. In a systematic review, it was reported that cystic transformation can be detected in some patients with BPH nodules [7]. In addition, it is possible that BPH nodules with cyst degeneration can be complicated by infection or hemorrhage [7]. Interestingly, our patient presented with gastrointestinal symptoms due to a rectal subepithelial cystic mass originating from the prostate gland without any urinary symptoms because the complicated cyst developed from an exophytic solid mass on the anterior side of the prostate that predominantly invaded into the rectal wall. Therefore, prostatitis might be considered as a rare cause of rectal subepithelial cysts, especially in elderly men.
In this case, the patient, who had BPH, experienced relapsed symptoms of difficulty in defecation with anal bleeding 1 year after surgery. Generally, BPH does not present with a symptomatic rectal subepithelial mass because the prostate gland is encapsulated by fibrous tissue [8]. However, the patient underwent surgical ablation for the complicated cyst, which might have weakened the rectal wall. In a previous report, rectal invasion of a prostatic mass could occur subsequent to an open transrectal biopsy of the prostate gland in patients with BPH [9]. Therefore, previous iatrogenic rectal wall injury could be a risk factor for rectal involvement of BPH mimicking direct invasion of prostatic cancer. BPH should be considered in the differential diagnosis of a rectal SMT in elderly men with a history of perirectal or transrectal procedures.
In this case, the rectal mucosa specimen obtained by endoscopic biopsy was not useful for evaluating whether the rectal SMT was malignant before the second surgical resection. The pathologic findings showing chronic active inflammation with pseudoepitheliomatous hyperplasia of the squamous epithelium could not exclude invasive prostatic cancer. In a previous report, endoscopic ultrasound-guided FNA/B (EUS-FNA/B) specimens determined the pathologic diagnosis of 5 rectal SMTs in 10 patients [10]. Although EUS-FNA/B was not performed because of the result of TRUS-FNA/B before the surgical ablation for the rectal subepithelial cystic mass, it might be a clinically useful method for the exact diagnosis and helpful for surgical planning of the removal of a symptomatic rectal SMT as in our case.
This is the first reported case of a patient with a benign prostatic mass mimicking a rectal SMT presenting as constipation and anal bleeding. BPH might be considered for the rare differential diagnosis of rectal SMTs in elderly men with the symptoms of constipation or anal bleeding, and who have a previous history of perirectal or transrectal procedures.