Neurogenic Bladder Dysfunction Caused by Seronegative Autoimmune Autonomic Ganglionopathy
Article information
Abstract
Autoimmune autonomic ganglionopathy is a form of acquired autonomic failure affecting parasympathetic, sympathetic functions, usually affecting healthy young people. The disorder affects both sympathetic and parasympathetic nervous systems, with acute onset, monophasic course, and partial recovery with relative preservation of motor and sensory function. We experienced a case of young man with acute autoimmune autonomic ganglionopathy who developed voiding difficulty, sudden blurred vision and gastrointestinal discomfort without motor or sensory dysfunction. Fever developed 5 days earlier and persisted until onset of autonomic failure. Patient complained voiding difficulty and urodynamic study revealed detrusor are flexia with failure to initiate and sustain adequate detrusor contraction. Sympathetic skin response and several autonomic function tests showed abnormalities. Intravenous immunoglobulin was applied for 5 days but symptoms persisted. Thus, 5 days of plasmapheresis treatment was followed showing improvements in most of the symptoms. However bladder dysfunction persisted at 6 months follow-up, showing partial recovery at bethanechol administration.
INTRODUCTION
Autoimmune autonomic ganglionopathy (AAG) is a form of acquired autonomic failure affecting parasympathetic and sympathetic functions [1]. Before the establishment of AAG as a disease entity, pure pandysautonomia with widespread autonomic failure affecting both cholinergic and adrenergic functions was referred to as acute pandysautonomia, acute panautonomic neuropathy, autoimmune autonomic neuropathy, and acute idiopathic pandysautonomia [2]. This pure pandysautonomia was first described as a distinct clinical entity by Young et al. [3] in 1969, known to usually affect healthy young people with an acute onset, a monophasic course, and partial recovery [4]. Recently, the discovery of serum antibodies of nicotinic acethylcholine receptor (AChR) on autonomic ganglia has led to a better understanding of this disorder, suggesting the involvement of immune mechanisms in idiopathic cases. Thus a new disease entity named AAG has been established [2].
Clinical features of AAG presents with combinations of nausea, vomiting, orthostatic hypotension, blurred vision, and urinary retention over 1 to 3 weeks [5]. The autonomic features of this disorder may involve both the sympathetic and parasympathetic nervous systems or the sympathetic or parasympathetic nervous system individually [4]. We experienced a rare case of acute AAG presenting persistent neurogenic bladder symptoms. Therefore, we report and review one recent case of young male patient with acute AAG.
CASE REPORT
A 30-years-old man with no previous medical history was admitted via the emergency room due to sudden blurred vision. Patient also complained of voiding difficulty at the time of admission. Before the admission, he developed an influenza-like illness with fever, headache, and myalgia. In neurological examination, patient’s consciousness was alert but his pupils were dilated to 5 mm bilaterally, and were unreactive to light. Extraocular muscle tests were intact and showed no signs of diplopia or facial palsy. Manual muscle test was intact, and showed no signs of hypesthesia or paresthesia in sensory nervous system. Deep tendon reflexes were decreased bilaterally at both lower extremities. There were no abnormalities in cerebellar function tests.
Examination of the cerebrospinal fluid specimen revealed no abnormalities such as oligoclonal bands and myelin basic protein. At laboratory studies, serum nicotinic AChR antibodies was negative, C-reactive protein was mildly elevated to 0.99 mg/dL but other laboratory studies including complete blood cell count, electrolytes, thyroid function tests, erythrocyte sedimentation rate, anti-ds-DNA antibodies and antibodies to cytomegalovirus, rubella virus, varicella zoster, herpes simplex virus, and Epstein-Barr virus revealed no abnormalities. Cranial computed tomography showed no brain lesion.
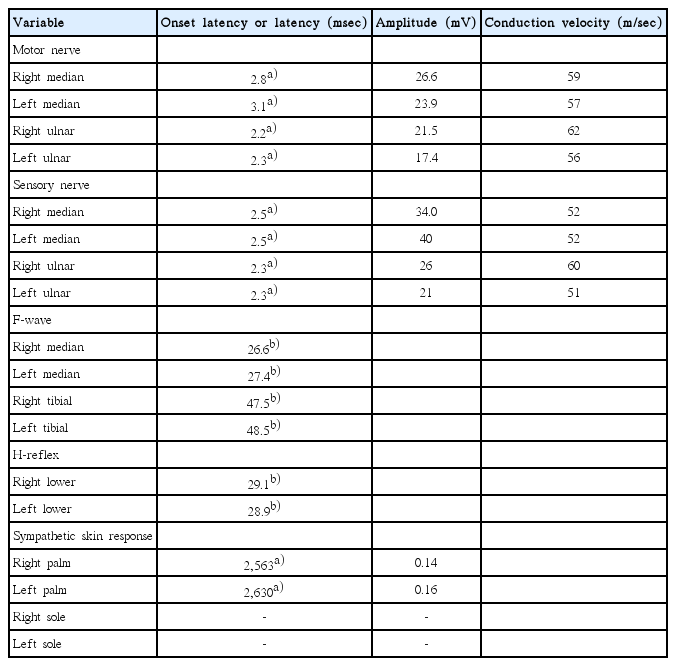
Because most symptoms of the patient were involving the autonomic nervous systems (absent pupil reflex, voiding difficulty, gastrointestinal discomfort, and hypohidrosis), autonomic function tests were performed the day after onset. Light reflex, accommodation reflex were all absent, and pupil showed dilatation from 5 to 7 mm in diameter 30 minutes after instillation of 1:1,000 epinephrine, and showed constriction from 5 to 3 mm in diameter 30 minutes after instillation of 0.125% pilocarpine. Blood pressure was decreased immediately at upright position, and was not able to test after 10 minutes standing since patient syncoped after standing for 7 minutes. Valsalva test and sweat response tests also showed impairments (Table 1). The findings of nerve conduction studies were normal, including normal proximal conduction (H-reflex). However, sympathetic skin response revealed decreased response at both upper extremities and absent response at both lower extremities (Table 2).
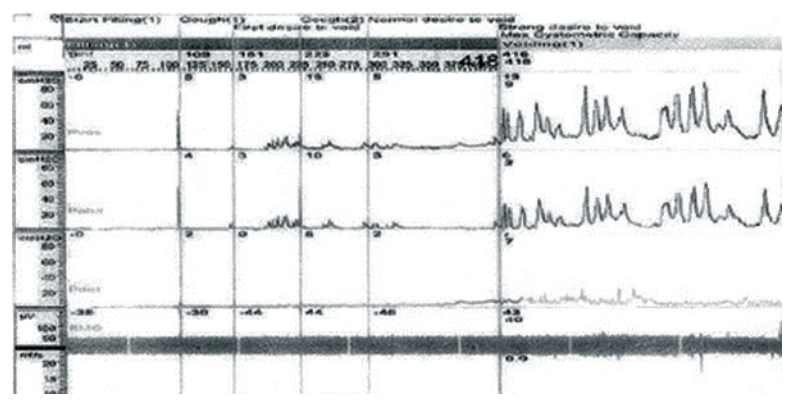
Because the patient could not void without intermittent bladder catheterization, urodynamic study was performed 14 days after onset using an urodynamic computer (Mediwatch UK Ltd, Rugby, UK). Patient showed decreased urinary flow at uroflowmetry with 200 mL of residual volume. Urodynamic study revealed detrusor areflexia with intact bladder sensation. Analysis of sphincter motor unit potential did not show any neurogenic abnormalities (Table 3, Fig. 1).
Initial urodynamic study revealed detrusor areflexia. Pves, intravesicular pressure; Pabd, abdominal pressure; Pdet, detrusor pressure; EMG, electromyography.
Despite the patient was treated with intravenous immunoglobulin from the first day of admission for five consecutive days, autonomic symptoms still persisted after 3 weeks. Thus, patient received intravenous fluid and nasogastric tube was placed for decompression and nutritional support. Then 5 days of plasmapheresis was followed from 25th day of onset. Blurred vision, hypohidrosis, and gastrointestinal discomfort improved after plasmapheresis treatment. Oral feeding was possible starting from 40th day of onset. Valsalva test and head-up tilt test showed improvements at 49th day of onset (Table 1). Patient was discharged after 51 days of onset.
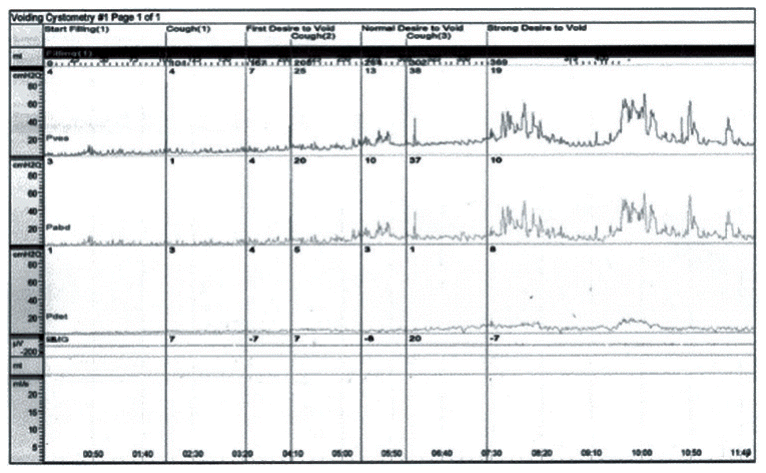
However, patient still could not void spontaneously, 25 milligrams of bethanechol was administered three times daily from 48th day of onset to improve the detrusor contraction. After administration of bethanechol, voiding difficulty improved partially. However, intermittent bladder catheterization was still necessary for three months. Bethanechol was continuously used until 6 months, and patient was able to void spontaneously although residual urine sense persisted. Follow-up urodynamic study was done at 191 days after onset, which still revealed detrusor areflexia (Table 3, Fig. 2). Most of other autonomic symptoms including gastrointestinal discomfort, hypohidrosis and the symptoms related to orthstatic hypotension improved completely.
DISCUSSION
Due to the etiology and process of acute onset, often with antecedent viral infection and monophasic course, AAG was first considered as the autonomic equivalent of Guillain-Barre syndrome [6]. The exact course and prognosis has not been well known, but according to the report by Camilleri [7], most symptoms recover at first year, then gradually improves during 4 to 5 years after onset.
Experimental evidence has shown that antibodies specific for the α3 subunits of ganglionic AChR which mediates synaptic transmission in peripheral autonomic ganglia, are found in many patients with acute or subacute AAG [8]. Also, the serum titer of the AChR antibody is known to be correlated with the severity of autonomic dysfunction [2]. However, serum nicotinic AChR antibodies were negative in our patient [8].
In treatment of AAG, the convincing evidence that AAG is an antibody mediated channelopathy supports the use of combined immunotherapies (immunosuppressive agents, intravenous immunoglobulin and plasmapheresis) [8,9]. Also conservative and acute management of hypotension, ileus and voiding difficulty is essential [2]. Our patient was initially treated with intravenous immunoglobulin but had minimal effects, thus plasmapheresis was followed, which showed significant effects in autonomic symptoms except for neurogenic bladder symptoms. This is in line with previously reported study by Winston and Vernino [8] which insisted that although seronegative AAG has no identified antibody marker, it is reasonable to consider autoimmune pathology based on reported response to immunomodulatory therapy.
Atonic bladder caused by AAG is a common feature also seen in diabetic polyneuropathy or Guillain-Barre syndrome, which supports the postganglionic type of pelvic nerve dysfunction affecting detrusor muscle contraction. Supersensitivity to minimal amount of bethanechol or carbachol was reported in previously reported cases, insisting that bladder muscle fibers maintain their ability to contract in response to agonist agents [10]. In our case, administration of bethanechol was started at 48th day of admission which showed decreased necessity of intermittent catheterization and decreased residual urine sense.
In previously reported cases, even though some micturition disturbance including voiding difficulty and nocturnal urinary frequency persisted, patients with urinary retention became to urinate within a week and micturition problems tended to resolve earlier than orthostatic hypotension, but this did not pertain to our case [10]. Due to delayed administration of bethanechol, overdistension injury might have been occurred leading to further detrusor muscle damage in our case, which leaded to persistent neurogenic bladder even after other autonomic symptoms were recovered.
In conclusion, we experienced a young male initially complaining several autonomic symptoms such as voiding difficulty, blurred vision, gastrointestinal discomfort, and hypohidrosis, who showed great recovery except for bladder symptoms. Thus, we report a rare case diagnosed as acute seronegative AAG with persisting neurogenic bladder dysfunction with review of the disease.