Food Protein-Induced Enterocolitis Syndrome in a Neonate with Peripheral Epimerase Deficiency Galactosemia
Article information
Abstract
Food protein-induced enterocolitis syndrome (FPIES) is a severe infantile form of non-immunoglobulin E-mediated gastrointestinal food hypersensitivity that manifests as profuse, repetitive vomiting, often with diarrhea, which leads to acute dehydration and lethargy and failure to thrive if chronic. Symptoms such as dehydration and lethargy are also observed in sepsis, viral infection, and food poisoning. It is difficult to differentiate FPIES from sepsis-like illness. The diagnosis is based on clinical criteria and/or an oral food challenge. FPIES developed in the patient with peripheral epimerase deficiency galactosemia after the use of soy formula. The change in feeding to soy formula is not required of a patient with peripheral epimerase deficiency galactosemia. Early intake of soy formula in our patient was harmful. Therefore, we think the changing the formula should be taken carefully. Another important point is the diagnosis. Late diagnosis and misdiagnosis are common, and inappropriate treatment or invasive treatment can occur.
INTRODUCTION
Food protein-induced enterocolitis syndrome (FPIES) is a severe infantile form of cell-mediated, non-immunoglobulin E (IgE)-mediated gastrointestinal food hypersensitivity that manifests as profuse, repetitive vomiting, often with diarrhea, which leads to acute dehydration and lethargy, or weight loss and failure to thrive if chronic [1].
Enterocolitis induced in infants by cow’s milk and/or soy protein has been recognized for decades [1]. Most children began to experience symptoms during early infancy (1 to 3 months but up to 1 year of age) within 1 to 4 weeks after introduction of cow’s milk or soy protein [2].
Galactose is metabolized in humans and other species by the three-enzyme Leloir pathway comprising the enzymes galactokinase, galactose 1-phosphate-uridylyltransferase, and uridine diphosphate-galactose 4′-epimerase (GALE). GALE deficiency galactosemia is called Epimerase deficiency galactosemia. Eimerase deficiency galactosemia is a continuum comprising three forms: generalized, peripheral, and intermediate. Peripheral type’s enzyme activity is deficient in red blood cells and circulating white blood cells, but normal or near normal in all other tissues. Infants with generalized type galactosemia develop clinical findings on a regular formula, but neonate with peripheral form remains clinically well even on a regular milk diet and only identified by laboratory findings [3].
In this case, we describe clinical and laboratory findings of a patient who has course of FPIES after being fed soy formula due to diagnosis of galactosemia. After all he is the patient was revealed as type of galactose epimerase deficiency not to need soy formula.
CASE REPORT
A male newborn weighting 3.0 kg (25th to 50th percentile) was delivered at 38+4 weeks of pregnancy by cesarean section. An additional galactosemia test was performed due to an abnormal result of the neonatal screening test. Galactose was measured by enzyme immunoassay and fluorophotometer. The result of galactosemia test showed an increased level of galactose-1-phosphate (galactose+galactose-1-phosphate: 33.56 mg/dL, normal level <9 mg/dL; galactose: 2.6 mg/dL, normal level <8 mg/dL; galactose-1-phosphate: 28.3 mg/dL, normal level <10 mg/dL). Hence, the newborn was put on a soy formula.
The neonate was admitted to the emergency center due to fever since 6 days and poor oral intake from the 23th day of life. There were no gastrointestinal symptoms such as diarrhea, vomiting, and bloody stools. Also the physical examination was completely normal. Physicians who were treating this infant for fever initially considered the possibility of infection. The neonate was diagnosed with septicemia on clinical examination and routine investigations, including complete blood count (CBC), C-reactive protein (CRP), blood culture, urine culture, and cerebrospinal fluid culture were performed. CBC showed a white blood cell (WBC) count of 16,270/mm3 (23% neutrophils, 61% lymphocytes, 10% monocytes, and 4% eosinophils). Anemia was also noted (hemoglobin [Hb] 9.9 g/dL, hematocrit [Hct] 29.1%). CRP level was elevated (9.63 mg/dL; normal range, 0 to 0.5 mg/dL). The results of blood culture and cerebrospinal fluid culture were negative. But urine culture was positive, and the organism identified was Enterococcus faecalis. Urine was collected through urine collector and urine analysis was normal. So we thought that result was shown because of contamination. Abdomen and pelvis ultrasonography demonstrated small and large bowel wall thickening, which is compatible with the diagnosis of enterocolitis. Therefore, the patient was diagnosed with enterocolitis and we thought that inflammatory markers were elevated due to enterocolitis. On the 5th day of admission, the neonate developed abdominal distension and diarrhea. Fever subsided on the 7th day. After the symptoms including diarrhea were improved, the infant was discharged.
On the first day after discharge, when the infant was 38 days of age, he developed grunting, perioral cyanosis, and persistent diarrhea. The mother stated that he was sleepy all the time. In the emergency department, vital signs were as follows: weight, 3.38 kg (<3rd percentile); height, 52 cm (5th percentile); head circumference, 38 cm (50th percentile); body temperature, 35°C; heart rate, 150 beats/min; respiratory rate, 34 breaths/min; and blood pressure, 71/50 mmHg. The presence of hypothermia was confirmed. On examination, the infant had an acute ill-looking appearance, pale skin, and dry lips. The remaining physical examination was normal.
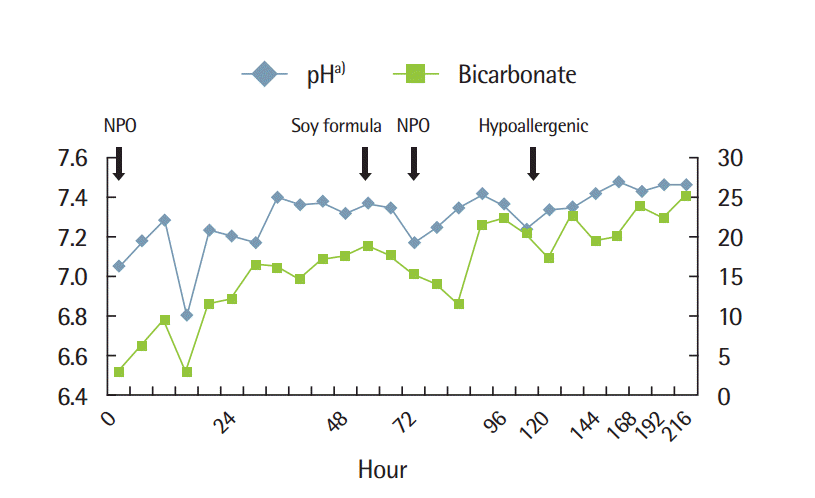
The laboratory studies revealed a WBC count of 54,170/mm3 (19% neutrophils, 61% lymphocytes, 7% monocytes, and 0% eosinophils), Hb and Hct were 10.0 g/dL and 29.6%, respectively, and platelets were 153,000/mm3. The CRP level was high (7.64 mg/dL). A capillary blood gas analysis revealed the following: pH 7.177, pO2 36.8 mmHg, pCO2 17.5 mmHg, and bicarbonate 6.3 mmol/L. Laboratory assessment also included comprehensive metabolic profile and stool examination, and the results were normal.
The patient was readmitted to our intensive care unit with a presumptive diagnosis of FPIES. But we could not rule out infectious or non-infectious disorders. Hence, treatment with antibiotics was initiated.
At that time, the neonate’s weight was 3.38 kg. There was a weight gain of 10 g per day. Sodium bicarbonate was started for correcting metabolic acidosis. For treatment of hyperammonemia (361 µg/dL; normal range, 29 to 70 µg/dL), the infant received nothing by mouth and was given intravenous fluids including glucose, and sodium benzoate was started. On hospital day 2, hyperammonemia improved (66 µg/dL; normal range, 29 to 70 µg/dL) and metabolic acidosis was corrected. A capillary blood gas analysis revealed the following: pH 7.377, pO2 39.7 mmHg, pCO2 29.7 mmHg, bicarbonate 17.1 mmol/L, and ammonia 66 μg/dL. The patient’s general condition and activity improved.
On hospital day 3, the soy formula, which had been given earlier was started (Soy; Namyang Dairy Products Co. Ltd., Seoul, Korea). Metabolic acidosis developed again within a day. The neonate was irritable and abdominal distension was observed. After this, the infant received nothing by mouth and was given intravenous fluids. Metabolic acidosis and symptoms were improved after fasting for one day. On hospital day 5, formula feedings containing extensively hydrolyzed milk proteins (HA; Maeil Dairy Industry Co., Seoul, Korea) were started. For the first time after at admission, the sucking reflex was observed, but the infant was not in a position to suck the milk. After several days, the neonate’s appetite increased, and capillary blood gas analysis parameters were stable after continuous feeding. On hospital day 6, repeat laboratory studies revealed hypoalbuminemia, severe anemia and thrombocytopenia (Hb 4.1 g/dL, Hct 12.5%, mean corpuscular volume [MCV] 88.0 fL, platelets 31,000/mm3 , total protein 3.2 g/dL, and albumin 1.5 g/dL). Abdominal and lower extremity edema secondary to hypoalbuminemia were observed. Hence, the neonate received red blood cell (RBC) concentrate, platelets, and albumin. On hospital day 8, physical examination showed reduction of the edema. The diarrhea disappeared and the infant did not show any further manifestations. The weight increased for 10 days by 320 g (3.7 kg [<3rd percentile], 32 g/day), and the infant was discharged home.
After discharge, the result of comprehensive metabolic profile including organic acid and amino acid analysis was normal. After 2 months, enzyme assay for galactosemia showed uridine diphosphate galactose-4-epimerase deficiency (4.0 μmoL/hr/g; normal range, 19-35 μmoL/hr/g). So we suspected peripheral epimerase deficiency galactosemia. The initial total IgE level and specific IgE level against milk and soy were low (total IgE 60.6 KU-L, neonate normal range <5 KU-L; milk: 3.28 μg/L, neonate normal range <0.35 μg/L; soy: 2.72 μg/L, neonate normal range <0.35 μg/L) [4,5]. When the infant was 6 months of age, the food-specific IgE levels were normal (total IgE 12.5 KU-L; milk <0.1 μg/L; soy <0.1 μg/L), and hence the clinicians started the food weaning process. After the infant was 7 months old, vegetables, beef, and soy were added to the diet, and he tolerated them well. Hence other foods were introduced progressively without any problems. After the extensively hydrolyzed milk protein formula was discontinued when the infant was 12 months old, soy, cow’s milk, and cheese products were introduced and he tolerated them well. Because the patient did not have hypotonia, poor feeding, vomiting, hepatomegaly, and liver dysfunction after general diet, the infant was diagnosed with peripheral epimerase deficiency galactosemia (Fig. 1).
DISCUSSION
FPIES is a severe infantile form of cell-mediated, non-IgE-mediated gastrointestinal food hypersensitivity that manifests as profuse, repetitive vomiting, often with diarrhea, which leads to acute dehydration and lethargy, or weight loss and failure to thrive if chronic [1].
Hypothermia may also be seen [2]. Symptoms such as acute dehydration and lethargy are also observed in sepsis, viral infection, and food poisoning. Hence, it is difficult to differentiate FPIES from sepsis-like illness or non-infectious disorders. Symptoms of FPIES usually begin in early infancy (1-3 months but up to 1 year of age) within 1 to 4 weeks after introduction of cow’s milk or soy protein [2].
Symptomatic infants improve promptly when they are given intravenous fluids or casein hydrolysate-based or amino acid-based formula [6]. Reintroduction of foods induces symptoms, including shock in 15% to 20% of cases [6]. During confirmatory oral challenges, symptoms develop at 1 to 10 hours after ingestion and are accompanied by an increase in the absolute neutrophil count in the peripheral blood [2].
The diagnosis is based on clinical criteria and/or an oral food challenge (OFC). FPIES criteria are proposed by Sicherer et al. [1], the diagnosis of FPIES is confirmed by an OFC and by the fact that the patients becomes asymptomatic following elimination of the suspected food [7]. If more than two of the following symptoms (vomiting, diarrhea, lethargy, and irritability) disappear and are induced within 24 hours of the diagnosis of FPIES is confirmed [6]. In our patient, metabolic acidosis and lethargy were also improved after fasting for 2 days. Although OFC is the standard criteria for the diagnosis of FPIES, infants usually do not need to undergo the confirmatory test if they have classic history and symptoms [2]. After the symptoms were improved and laboratory findings returned to normal, the patient which was used before. Within a day, metabolic acidosis recurred and his general condition deteriorated. Physicians thought that FPIES was induced due to the incidental OFC test. The symptoms resolved after removal of the offending food from the patient’s diet within 24 hours. We could finally diagnose FPIES after the patient’s symptoms and acidosis were improved, and they were induced due to administration of the soy formula.
Parameters for early recognition of FPIES are weight change (weight [g]-birth weight [g]/day), serum albumin level, peripheral leukocyte count, metabolic acidosis, peripheral eosinophilic count, peripheral platelet count, and stool examination (occult blood, leukocytes, and red cells) (Table 1) [8]. The inclusion criteria were as follows: age 15 to 45 days [8]. The failure to gain weight was defined as a daily weight gain of <10 g. A serum albumin level of <3.5 g/dL was considered as a low serum albumin level. A WBC count of >19,500 cells/mm3 was considered as peripheral blood leukocytosis, and metabolic acidosis was diagnosed when the serum bicarbonate level was <22 mEq/L. Eosinophilia was defined as >500 cells/µL, and peripheral blood thrombocytosis was defined as >750,000 cells/mm3 [1,9]. An abnormal stool smear test was defined as positive for occult blood and/or WBC, RBC >5/HPF [7]. In our patient, hypoalbuminemia and metabolic acidosis were noted. Also there was a weight gain of 10 g per day. Despite the fact the patient’s weight gain was no less than 10 g per day, it was not an adequate amount either. A failure to gain weight represents a failure to thrive. Failure to gain weight and serum hypoalbuminemia are the highest indexes of suspicion for FPIES [8]. The results also showed peripheral blood leukocytosis. Although leukocytosis is associated with FPIES, leukocytosis is also observed in neonates or infants with sepsis-like illness. Hence leukocytosis does not provide a high index of suspicion of FPIES [8]. And in our patient, anemia was also noted. At admission, Hb and Hct were normal. But on hospital day 6, repeat laboratory studies revealed decreased Hb and normal MCV. So anemia was thought due to acute blood loss. In FPIES, this result is rare.
OFC is the gold standard for diagnosing FPIES [10]. But, the infant in our case did not undergo confirmatory challenges because he had a classic history of severe reactions and he became asymptomatic following elimination of the milk and soy. OFC in FPIES is considered a high-risk procedure because 50% of the reactive challenges require intravenous hydration [10].
Clusters of eosinophils have been found in intestinal biopsy specimens from many patients with FPIES [2]. But our patient did not undergo intestinal biopsy. Patients with FPIES show negative allergy tests (specific-IgE and/or skin-prick testing) [11]. Total IgE and specific-IgE levels against milk and soy in our patient were not adequate to indicate IgE-mediated food allergy (total IgE 60.6 KU-L, neonate normal range <5 KU-L; milk: 3.28 μg/L, neonate normal range <0.35 μg/L; soy: 2.72 μg/L, neonate normal range <0.35 μg/L), but the result indicated a moderate level, grade 2. Meaningful predictive decision points may help to avoid oral food challenges. However, data need to be ascertained for each neonate [12]. Atypical cases have been described with detectable specific IgE to the causal protein and with a more prolonged course of allergy [2]. Hence we thought that the clinical course of this infant was severe due to the atypical type of FPIES.
After discharge, enzyme assay for galactosemia showed uridine diphosphate galactose-4-epimerase deficiency. Because the patient did not have hypotonia, poor feeding, vomiting, hepatomegaly, and liver dysfunction after general diet, the infant was diagnosed with peripheral epimerase deficiency galactosemia. Infants with peripheral epimerase deficiency galactosemia do not need any treatment. Breast–fed infants are considered to be at a lower risk for developing protein hypersensitivity. Early intake of soy formula in our patient was harmful.
The prognosis of FPIES is good. A retrospective study involving patients with FPIES reported resolution occurs within 2 years in 60% of patients with cow’s milk sensitivity and in 25% of patients with soy milk sensitivity [6]. In this infant, after 9 months of age, the unicap test was normal, and hence we stopped the formula containing extensively hydrolyzed milk proteins. Vegetables, beef, and soy were added to the diet early, and he tolerated them well; other foods were also introduced progressively without any problems.
Acknowledgements
This work was supported by the Soonchunhyang University Research Fund.