Acute Kidney Injury Arising from Severe Hyperemesis Gravidarum: Case Report with a Review of Literatures
Article information
Abstract
Severe hyperemesis gravidarum is a rare but potentially life-threatening it left untreated. Its serious complications are dehydration, ketosis, alkalosis from loss of hydrochloric acid, hypokalemia, and compromised pre-renal acute kidney injury. We experienced a very rare case of a 20-year-old woman who presented to the emergency department with severe hyperemesis gravidarum associated with a loss of kidney function at 25 weeks’ gestation. Her initial serum creatinine and blood urea nitrogen were 5.0 and 45.9 mg/dL, respectively. The patient underwent hemodialysis for three days and achieved a subsequent recovery of renal function. In conclusion, our case indicates that clinicians should be aware of the possibility of acute kidney injury associated with severe hyperemesis gravidarum although rare.
INTRODUCTION
Acute kidney injury (AKI), also known as acute renal failure, is an abrupt loss of renal function, and it occurs within seven days of the onset of injury [1]. It may occur secondary to hypotension due to severe hyperemesis in pregnant women who were diagnosed with oliguria (urine output <400 mL/day) and mounting azotemia (serum creatinine >2 mg/dL) despite a lack of specific disease history [2].
We experienced a very rare case of a 20-year-old woman who presented to the emergency department with severe hyperemesis gravidarum associated with a loss of kidney function at 25 weeks’ gestation. The patient underwent hemodialysis (HD) for three days and achieved a subsequent recovery of renal function.
CASE REPORT
A 20-year-old, primiparous woman presented to our emergency room with a history of intractable nausea, vomiting and dizziness at 25 weeks’ gestation. These symptoms occurred since 9 weeks’ gestation and then were aggravated over seven days. The patient had a decrease in urine volume to nearly zero over 24 hours. The patient also had a 20-kg weight loss for the past four months. The patient had no notable findings on family and past medical history including renal, hepatic, and thyroid function at antenatal examination. In addition, the patient also had no history of psychiatric disease, eating disorder, or drug abuse. The patient underwent uneventful course until 9 weeks’ gestation. Then, the patient had a body weight of 80 kg. Moreover, the patient presented with emesis since 9 weeks’ gestation. Nevertheless, the patient had aggravation of symptoms which were unresponsive to dietary modification, antiemetics and antacids.
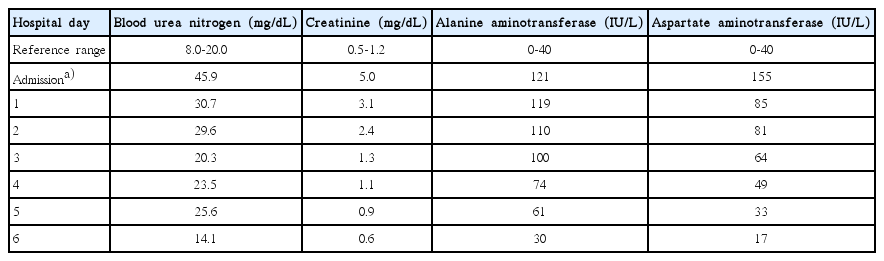
On physical examination, the patient had edema of both lower legs, accompanied by unstable vital signs. That is, the patient had a body weight of 60 kg, a blood pressure of 80/50 mmHg, a pulse rate of 130 beats/min, a respiratory rate of 20 breaths/min, and a body temperature of 37°C. On serum biochemistry, the patient showed sodium 125 mEq/L (reference range, 136 to 145 mEq/L), chloride 72 mEq/L (reference range, 96 to 110 mEq/L), potassium 2.2 mEq/L (reference range, 3.5 to 5.1 mEq/L), and bicarbonate 36.1 mEq/L (reference range, 21 to 31 mEq/L). The patient underwent insertion of Foley catheter, thus yielding a 5 mL of urine over one hour. On microscopic urine analysis, the patient showed 10 to 19 red blood cells, 30 to 49 white blood cells, proteinuria +1, trace ketones, 5 to 9 epithelial cells, and 1 to 2 casts. On arterial blood gas analysis, the patient showed pH 7.574 (reference range, 7.35 to 7.45), pCO2 46.2 mmHg (reference range, 32.0 to 45.0 mmHg), bicarbonate 41.8 mmol/L, and base excess 17.9 mmol/L. Moreover, the patient also showed hemoglobin was 11.3 g/dL (reference range, 12.0 to 16.0 g/dL), platelet 261/mm3 (reference range, 130 to 400/mm3 ), C-reactive protein 7.35 mg/L (reference range, 0 to 2.5 mg/L). Coagulation profiles were within normal limits. The patient was negative for hepatitis B, syphilis, and human immunodeficiency viruses. Moreover, the patient showed serum levels of thyroid stimulation hormone (TSH) 0.088 μIU/mL (reference range, 0.27 to 5.0 μIU/mL), and free thyroxine (free T4) of 2.92 ng/dL (reference range, 0.93 to 1.7 ng/dL). The patient showed blood urea nitrogen (BUN) of 45.9 mg/dL (reference range, 8.0 to 20 mg/dL), creatinine (Cr) of 5.0 mg/dL (reference range, 0.5 to 1.2 mg/dL), uric acid of 22.4 mg/dL (reference range, 3.0 to 7.0 mg/dL), total bilirubin of 1.3 mg/dL (reference range, 0.2 to 1.2 mg/dL), alanine aminotransferase (ALT) of 121 IU/L (reference range, 0 to 40 IU/L), and aspartate aminotransferase (AST) of 155 IU/L (reference range, 0 to 40 IU/L). Finally, the patient was positive for anti-thyroglobulin and anti-microsomal antibodies.
Fetal estimated weight was 710 grams, which is compatible with 25 weeks’ gestation. The patient also had normal amniotic fluid volume on transabdominal ultrasound. Despite a massive intravenous hydration, the patient had oliguria for three hours. We therefore determined to perform HD. The patient underwent insertion of catheter in the femoral vein. Dialysis was begun within three hours of admission, and it lasted for three days. The patient achieved improvement in BUN, Cr, ALT, and AST (Table 1).
On hospitalization day 6, the patient was discharged from us after receiving antiemetics. One week later, the patient received follow-up laboratory examinations. This showed that the patient achieved a recovery of renal and hepatic functions. Two weeks later, without anti-thyroid treatment, the patient also achieved normal values of serum TSH and free T4 levels. At 38 weeks’ gestation, the patient delivered a normal 2,700-g female infant.
DISCUSSION
It is known that pregnant women commonly exhibit mild to moderate nausea and vomiting until 16 weeks, and these symptoms are frequently self-limited. According to a review of literatures, however, 0.5% to 1% of total pregnant women present with severe nausea and vomiting accompanied by the decreased body weight by 10% or more [3].
It is presumed that multifactorial characteristics are involved in the pathogenesis of hyperemesis. Presumably, it might be associated with high serum levels of pregnancy related hormone, such as estriol, progesterone, human chorionic gonadotropin, leptin, thyroxine, and ghrelins [4,5]. The complications of severe hyperemesis include dehydration, hypotension, electrolyte imbalance, Wernicke encephalopathy, and hepatic and renal dysfunctions. This may be fatal to both mother and fetus. There are some reports about AKI arising from hypotension due to severe hyperemesis [6,7]. In the current case, the patient had no history of kidney disease by 9 weeks’ gestation. This suggests that the patient presented with AKI arising from hypotension due to severe volume depletion in association with severe nausea and vomiting. There are also some cases of transient hepatic dysfunction accompanied by the accumulation of biliary sludge [8]. In addition, women with hyperemesis are vulnerable to lower serum TSH and higher free T4 levels [9]. It is generally known that hepatic dysfunction and derangements in total bilirubin and serum free T4 levels are quickly normalized after hydration. Our patient also developed transient hepatic dysfunction and hyperthyroidism. But the patient achieved a recovery of hepatic dysfunction and total bilirubin levels immediately after hydration and HD. Two weeks after discharge, the patient also achieved a normal values of serum TSH and free T4 levels without treatments. The optimal timing and frequency of HD remain unclear. According to some studies, however, early and intensive HD might be effective in improving renal functions and maternal and fetal survival [7]. This explains why we performed HD within three hours of admission. Thus, we obtained good treatment outcomes. The parenteral nutrition may be needed for a number of women who had a persistent presence of intractable vomiting [10]. It has been reported that patients with severe hyperemesis are at increased risk of developing placental dysfunction disorders such as placental abruption, small for gestational age, and preeclampsia [11].
In conclusion, our case indicates that clinicians could not completely rule out the possibility that pregnant women with severe hyperemesis should be suspected of having prerenal AKI. In this case, it would be mandatory to perform early and intensive HD, which is essential for improving both renal functions and pregnancy outcomes.