Thebesian vein Combined with Apical Hypertrophic Cardiomyopathy
Article information
Abstract
A 52-year-old woman presented with atypical chest pain. Coronary angiography demonstrated multiple microfistulae between left coronary artery and left ventricle (LV) cavity, extensive enough to produce an LV angiogram. This LV angiogram revealed apical hypertrophic cardiomyopathy (HCM) which was confirmed by echocardiography. Coronary steal phenomenon by coronary artery microfistulae and HCM might have a role for developing of angina in patient with apical HCM.
INTRODUCTION
Coronary artery fistulae (CAF) is an unusual anomaly and a predominantly congenital communication between the coronary arterial system and the chambers or great vessels of the heart. Generally CAF presents as a single fistula terminating into the right heart or the pulmonary trunk [1]. Thebesian veins were discovered by Raymond Vieussens and Adam Christian Thebesius nearly three centuries ago. This is a very rare form of coronary fistula which multiple communications exist between coronary arteries and the left ventricle [2]. Fetal regression of this structure results in the formation of the Thebesian vessels of the adult heart. Thus, interference with developmental changes might produce an abnormally prominent Thebesian system with morphological appearance of multiple coronary microfistulae [3,4].
CASE REPORT
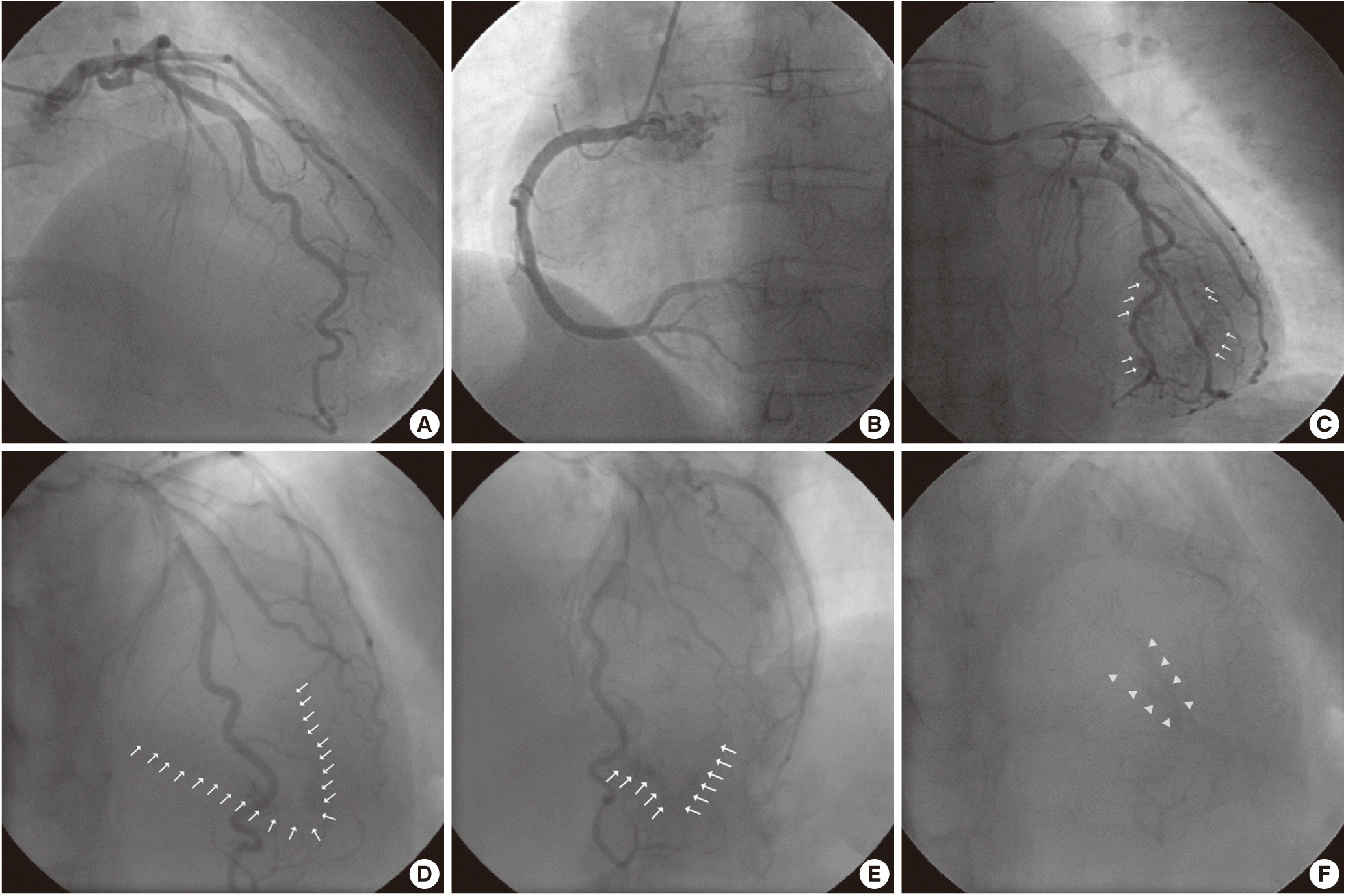
A 52-year-old woman, who had diabetes, hypertension, and dyslipidemia, was admitted with atypical chest pain. Her electrocardiogram revealed giant negative T-wave on precordial leads (Fig. 1A). Coronary angiography was performed in order to exclude ischemic heart disease. The left coronary artery and right coronary artery showed no significant stenosis (Fig. 2A, B). Left coronary injection demonstrated a capillary blush draining into the left ventricular (LV) cavity through multiple microfistulae (Fig. 2C) all along the wall of the left ventricle, extensive enough to produce an LV angiogram (Fig. 2D, E). During systole, LV cavity was almost obliterated (Fig. 2F). The appearance suggested arterioluminal-type Thebesian veins communicating between the coronary arteries and LV cavity. This LV angiogram revealed spade shaped LV apex which is hallmark of apical hypertrophic cardiomyopathy (HCM). It was confirmed by echocardiography (Fig. 1B). Her chest pain was improved after conservative treatment.
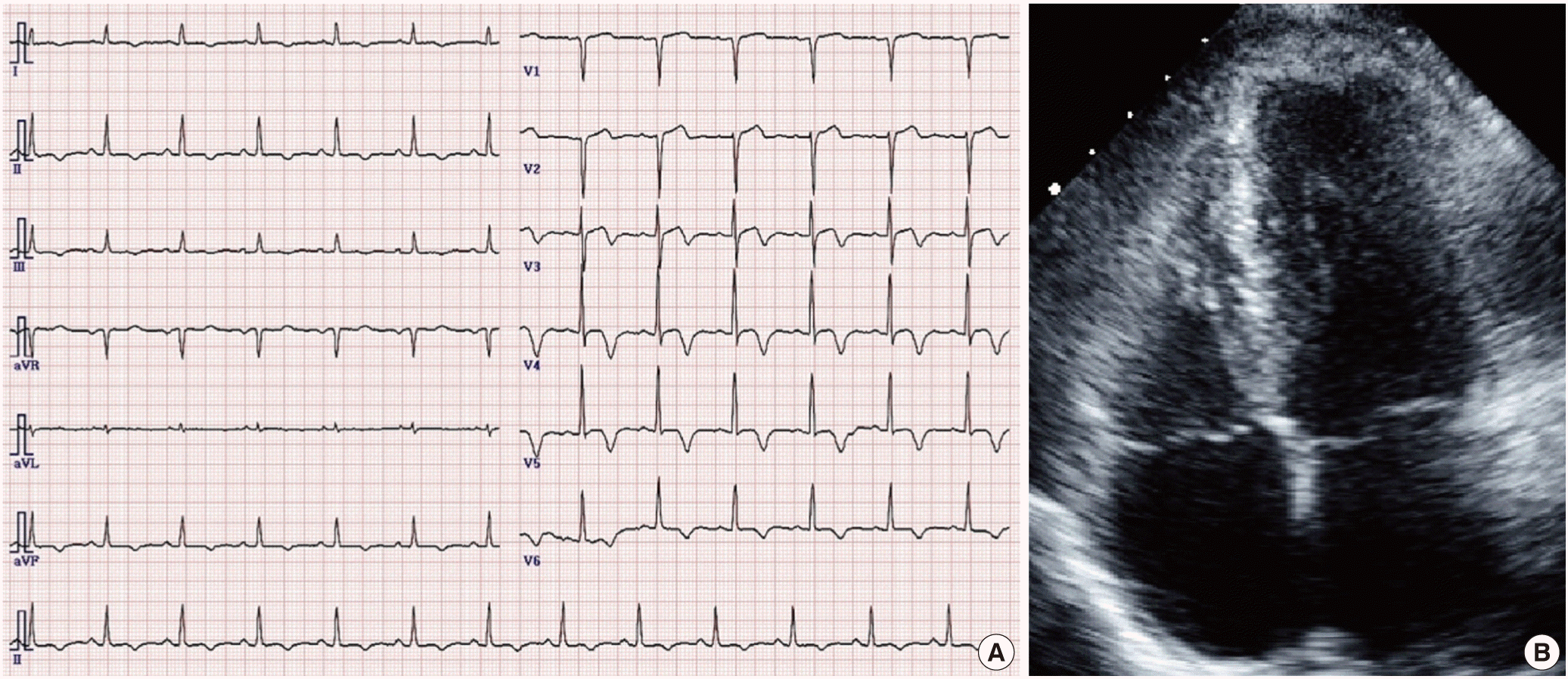
(A) Electrocardiogram shows giant negative T-wave on precordial leads. (B) 2-dimensional echocardiography (apical 4 chamber view) shows apical hypertrophic cardiomyopathy.
DISCUSSION
It has been hypothesized that coronary artery left ventricular fistula (CALVF) most likely represents partial persistence of the network of Thebesian veins that provide blood supply to the subendocardial regions of the myocardium during embryogenesis and are usually obliterated in normal development [5]. Congenital CAF have been reported in 0.08% to 0.3% of 126,595 adults undergoing coronary angiography [6]. Thebesian veins are believed to have two separate functions. First, they provide a route of myocardial drainage. Second, they offer an alternative route of nourishment to the myocardium [7]. Thus, CALVF is considered as a possible cause of exertional angina. Three types of Thebesian veins of the ventricles have been described. The first type of Thebesian veins drain blood from the capillary bed into the ventricles. The second type, arterioluminal vessels, drain blood directly from arteries into the ventricles without traversing capillary beds. The third type, the venoluminal vessels, form direct connections with the coronary veins, shunting blood from these vessels into the ventricles [8].
In most cases of CALVF, patients are asymptomatic and the fistulas are coincidentally found [9]. However, anginal symptoms have frequently been described. Myocardial ischemia could result from a shunt-related coronary steal, due to the diversion of oxygen-rich blood into the LV cavity via the low-resistance fistulous channels bypassing the myocardium, or a critical drop in perfusion pressure because of rapid run-off and impaired subendocardial perfusion of the hypertrophied myocardium [10].
Thebesian vein with apical HCM has been rarely reported. According to these literatures, hypertrophic cardiomyopathy and coronary artery–cardiac chamber fistulae could simply co-exist or there could be a causative relationship between the two conditions. However, the pathophysiological background remains unclear [3,11,12]. The main mechanism of myocardial ischemia related to coronary artery–LV fistulae is the coronary steal phenomenon. Nevertheless, HCM can also cause ischemia by altering the oxygen demand/supply balance to the hypertrophic myocardium. Since both coronary artery fistulae and HCM can produce myocardial ischemia and angina [13]. In conclusion, these mechanisms might have a role for developing of angina in this patient.