Characteristics of Device-Associated Cerebrospinal Fluid Infection in Adults
Article information
Abstract
Objective:
Device-associated infections in the central nervous system are serious complications of procedures involving indwelling devices among neurosurgical patients. In this study, the clinical characteristics and outcome of microbiologically confirmed deviceassociated cerebrospinal fluid (CSF) infection were evaluated.
Methods:
We performed a retrospective analysis of adult patients found to have a positive CSF culture result during a hospital admission between 1 January 2005 through 2 October 2010 in Soonchunhyang University Hospital.
Results:
During the study period, all episodes (n=161 CSF specimens, 87 patients) involving a culture-positive CSF were enrolled. Thirty-two episodes of device-associated CSF infection were included in the analysis among the study group. Most device-associated infections were ventriculo-peritoneal shunt infections (14/32, 44%). Fever (>38°C) was present in 17 episodes (53%). Overall, the most common microorganism was coagulase-negative staphylococcus (7/32 [22%]). Gram-negative rods (Pseudomonas aeruginosa 6/32 [19%], Acinetobacter baumannii/haemolyticus 5/32 [16%]) were identified in culture in 16/32 (50%). Device was removed for the control of device-associated infection in 30/32 (94%). Cure rate was 69% (22/32). All patients with treatment failure (10/32, 34%) expired.
Conclusion:
It is difficult to diagnosis device-associated CSF infections early since those are frequently presented with nonspecific clinical signs and symptoms. In our study, gram-negative infections accounted for 50% of cases and the empiric antibiotics initially chosen were found to not be effective against the final identified pathogen in many cases. Device-associated CSF infections should be strongly considered a serious risk factor associated with CSF infections, and prompt initiation of broad coverage antibiotics should be started after appropriate assessment.
INTRODUCTION
Device-associated infections of the central nervous system among neurosurgical patients include infectious complications of procedures such as intraventricular shunt operations, external ventricular drainage, external lumbar drainage, or cerebrospinal fluid (CSF) reservoir placement. A better understanding of device-associated infections is limited by the heterogeneity of cases (e.g., various neurosurgical procedures devices, different populations, etc.). Based on a recent literature review, the incidence of shunt–associated, external ventricular drainage-related, lumbar drainage-related, and Ommaya reservoir-related infections were found to be 1% to 18%, 2% to 27%, 7%, and 15%, respectively [1-8]. Three of these studies used a clinical diagnosis to describe the characteristics of infection. We performed a retrospective analysis focused on episodes of microbiologically confirmed device-associated CSF infections in adult patients. In our study, we attempted to describe the clinical and microbiological characteristics and outcome of culture proven device-associated CSF infection.
MATERIALS AND METHODS
This retrospective study was conducted at a 705-bed tertiary hospital using medical records and the hospital’s electronic laboratory database of adult patients admitted from 1 January 2005 through 2 October 2010. All cases with positive CSF culture results during the study period were identifed. We considered each individual case as a single episode if identical bacterial organisms were recovered more than once in the same patient within 14 days.
Based on the medical records, we reviewed the cases involving patients meeting the criteria for a device-associated infection. A thorough review of each enrolled cases’ microbiological and clinical characteristics was examined. Collected data included the type of implanted CSF device, the day of insertion/removal, underlying characteristics, purpose of device insertion, clinical signs of early meningitis, antibiotics regimen utilized, duration of treatment, CSF laboratory findings, findings of brain imaging, and the clinical outcomes.
1. Definition and exclusion criteria
‘Devices’ were limited to medical devices used in 4 neurosurgical procedures: intraventricular shunt operations, external ventricular drainage, external lumbar drainage, or CSF reservoir. Device- associated CSF infection refers to episodes related to devices within the CSF space or within 48 hours after removal. Device-associated CSF infection were defined using the Center for Disease Control (CDC) criteria [9] for nosocomial bacterial meningitis. The criterion is based on laboratory findings and clinical information and should include ≥1 of the following laboratory values: CSF white blood cell (WBC) count of >10 cells/mm3, CSF protein level of >45 mg/dL, and CSF glucose level of ≤50% of the serum glucose level (if serum glucose level was not checked, glucose level ≤40 mg/dL).
We excluded patients who were given a diagnosis of meningitis or ventriculitis at the time of device insertion, and those not meeting the ‘device-associated’ infection criteria (e.g., positive CSF culture result after 48 hours of device removal.). Positive culture results not meeting the CDC criteria were considered as contamination or catheter colonization.
‘Cure’ was defined as disappearance of all signs and symptoms of meningitis and sterilization of CSF culture within 30 days. ‘Failure’ was defined as neither disappearance of all signs and symptoms of meningitis nor sterilization of CSF cultures within 30 days, or a relapse within 30 days. Device-associated infection related death was defined as death without an alternative cause of death. ‘Contamination’ was defined as an isolated positive CSF culture in the absence of abnormal CSF findings.
2. Statistical analysis
Descriptive statistics such as mean, median, and range were used to describe baseline demographic and clinical profile of all patients’ data. Fisher’s exact test was used to compare mortality between the gram-negative bacterial infection and gram-positive bacterial infection groups. A P-value of less than 0.05 was considered as significant. All the data analyses were performed using the statistical software package SPSS ver. 14.0K for windows (SPSS Inc., Chicago, IL, USA).
RESULTS
1. Demographic and characteristics of patient with deviceassociated cerebrospinal fluid infection
All episodes (n=161 CSF specimens, 87 patients) with culturepositive CSF for the study period were identified. Among 161 episodes, only 48 episodes (35 patients) involved culture-positive CSF associated with neurosurgical devices in the CSF space or involved cases where a device was removed within 48 hours. Among the 48 episodes, 32 episodes (26 patients) met the criteria of the deviceassociated CSF infection and included in the analysis. The others of episodes were 1 postoperative meningitis and 15 contaminations.
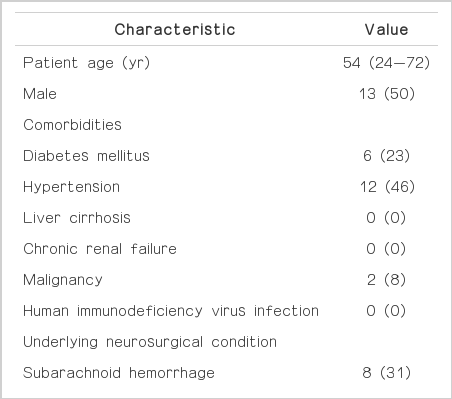
The characteristics of the 26 patients categorized as having a device- associated CSF infection were as follows; the median age was 54 years (range, 24 to 72 years); 13/26 (50%) were male. The most common medical comorbidity was hypertension (12/26, 46%). The most common underlying neurosurgical condition was subarachnoid hemorrhage or intracerebral hemorrhage (16/26, 62%) (Table 1). The main reason for device insertion was due to communicating hydrocephalus (24/32, 75%). Most device-associated infection was ventriculo-peritoneal shunt infections (14/32, 44%) (Table 2).
2. Clinical characteristics
Table 2 lists the neurological signs and symptoms at the time of device-associated CSF infection diagnosis. Fever (>38°C) was present in 17 episodes (53%), whereas headache (13%), nausea (9%), neck stiffness (3%) or decrease of Glasgow coma scale (19%) were less common. Local signs of infection were present in 3 episodes (9%) and included erythema, local pain, swelling, and/or purulent wound discharge. Nine episodes (28%) did not have fever or neurological signs or local manifestations of infection. Among those episodes, the most common pathogen was coagulase-negative staphylococci (3/9, 33%).
3. Radiological characteristics
The cranial computed tomography (CT) scan was checked in 27 episodes. The CT scan showed findings of ventriculitis in 7% (2/27) and hematoma in 7% (2/27) of episodes. Meningitis or brain abscess was suspected in 15% (4/27). Among the 27 episodes, 8 (30%) had cranial CT findings compatible with meningitis.
4. Laboratory characteristics
CSF pleocytosis (median, 75 cells/mm3 [range, 2 to 15,120]) was the most frequent (26/32, 81%) laboratory finding (Table 2).
5. Microbiologic findings
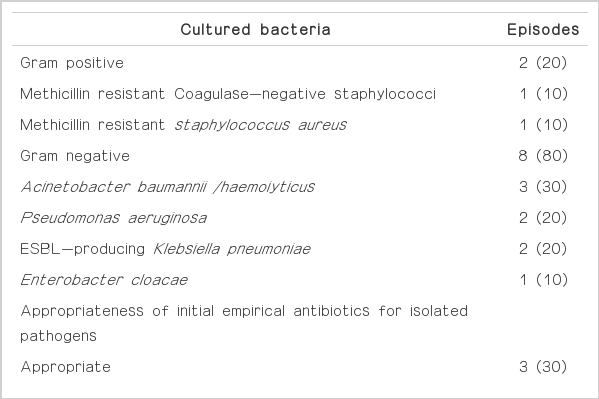
Overall, the most prevalent organism was coagulase-negative staphylococcus (7 [22%] of 32 episodes). Gram-negative rods were identified by culture in 16 (50%) of 32 episodes (Table 3).
6. Antimicrobial and surgical treatment
In most episodes (31/32, 97%), patients received systemic antibiotic treatment. However, the initial antibiotic choice was inappropriate for giving susceptibility results of isolated pathogen in 56% (18/32) the episodes. Device removal decision was made along with the clinical condition or response to antibiotic treatment. Devices were removed to control device-associated infection in most cases (30/32, 94%). Device removal was not performed in 2 cases because of good clinical and laboratory response to initial antibiotic treatment (Table 4).
7. Outcome
Table 4 shows the treatment outcome at last follow-up. The cure rate was 69% (22 of 32 episodes). All patients with treatment failure (10/32, 31%) died from device-associated infection. Most patients (8/10, 80%) with treatment failure had gram-negative bacterial infection (Table 5). The mortality rate tended to be higher in patients with gram-negative bacterial infection than in those with gram-positive bacterial infection (P= 0.05). Multidrug-resistant pathogen was isolated in 5 patients: multidrug-resistant Acinetobacter baumannii/haemolyticus in 3 patients; extended-spectrum β-lactamase (ESBL) producing Klebsiella pneumoniae in 2 patients. In 10 episodes with device-associated infection related death, initial empirical antibiotics were inappropriate in 7 (70%).
DISCUSSION
Device associated meningitis and ventriculitis represent possible life-threatening conditions that may lead to a permanent adverse outcome in neurocritical care patients. It is difficult to diagnose these infections early because they often present with nonspecific clinical signs, and affected patients can have normal CSF leukocyte counts. There are no definite acute phase parameters for the differential diagnosis of ventriculitis in neurocritical care patients [10].
Sundbarg et al. [11] classified a positive CSF culture results as a ‘definite ventriculostomy-related infection’ if it was associated with CSF pleocytosis (defined as at least 11 leukocytes/mm3 with 50% or more polymorphonuclear neutrophils) and clinical symptoms that could not be attributed to causes other than ventriculitis. In our study, among CSF culture-positive episodes with neurosurgical devices, the majority of episodes (32/48, 66%) were deviceassociated CSF infection. Similar to the findings of a retrospective analysis of 78 cases in Switzerland [10], our study showed that device- associated infections often presented with nonspecific clinical signs and symptoms. Twenty eight percent of patients had no fever and no neurological abnormalities. Among laboratory data, cell index (>5), CSF-to-blood glucose ratios (≤0.5), pleocytosis (WBC >10 cells/mm3), and CSF total protein levels (>45 mg/dL) had relatively high correlations than CSF glucose levels (≤40 mg/ dL).
Common bacterial pathogens of CSF catheters are coagulasenegative Staphylococci (especially staphylococcus epidermidis), staphylococcus aureus, facultative and aerobic gram-negative bacilli (including Pseudomonas aeruginosa), and Propionibacterium acnes [12]. Traditionally, gram-positive microorganisms are the most common pathogens that cause intraventricular catheter related ventriculitis [13]. However, recent studies report a preponderance (range, 51% to 82%) of gram-negative infections [14,15], which are associated with a higher mortality rate (58%) in some studies [16]. Especially, acinetobacter spp. infection after neurosurgery is becoming an increasingly. It is related with high mortality due to its resistancy for carbapenem (72.5%) [17]. Our study also showed gram-negative rods in 50% of the device-associated CSF infections and they are associated with a higher mortality rates. And our result also showed highly proportion of resistant pathogen. 60% (3/5) of Acinetobacter baumannii/haemolyticus was carbapenem resistant. And all isolated Klebsiella pneumonia was ESBL producing-pathogen. Five patients with CSF infection due to this multidrug-resistant pathogen died.
Furthermore, the initial empiric antibiotic choice was inappropriate in many cases (18/32, 56%) due to increased gram negative bacteria (14/18, 88%) and multidrug-resistance (5/18, 28%). Therefore, broad spectrum antibiotics should be initially administered to patients at risk of infection caused by multidrug-resistant gramnegative bacteria.
Early recognition and aggressive treatment with coverage for gram-negative organisms and multidrug-resistant pathogens may improve the outcome for patients with device-associated CSF infections.
In conclusion, our results show the increased incidence of gramnegative bacteria as a causative pathogen associated with deviceassociated cerebrospinal fluid infection. To improve survival, it is necessary to make a timely diagnosis and initiate appropriate antibiotics according to risk for infection caused by multidrug-resistant pathogens. Given that the initial empiric antibiotic was inappropriate in most cases, a guideline accounting for the current epidemiology of etiologic bacteria in the context of changing empirical regimens is required.