Diagnosis of a Malignant Tumor in a Patient with an Adnexal Mass Using Endoscopic Ultrasound Elastography
Article information
Abstract
Elastography is an imaging modality for the evaluation of tissue stiffness, which has been used for the analysis of superficial organs, such as those of the breast and prostate. The measurement of tissue elasticity has been reported to be useful for the diagnosis and differentiation of tumors, which are stiffer than normal tissues. Endoscopic ultrasonography elastography (EUS-EG) is a promising imaging technique with a high accuracy for the differential diagnosis of solid pancreatic tumors. However, to date EUS-EG has not been used to provide complementary information for biologic behavior of adenxal mass. We report our experience of EUS-EG in a patient with adnexal mass.
INTRODUCTION
Ovarian cancer has the highest mortality of all gynecologic cancers, despite representing only 3% of all cancers in women. It is the second commonest gynecological cancer, next to uterine cervix cancer, in Korea. Ovarian cancer incidence rate was similar to that in women worldwide but lower than those in Western countries, and the trend has been increased steadily [1]. Ovarian cancer-related mortality rates have been increasing in Korea [1]. Therefore differential diagnosis of ovarian cancer is very important in patients presenting with adnexal masses diagnosed either by physical examination or as a result of other imaging tests. Currently a variety of radiological imaging modalities can be used to characterize the tissues of ovarian malignancies, including ultrasound, computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET)/CT.
Elastography is an imaging modality for evaluating tissue stiffness, which is used to analyze superficial organs such as the breast and prostate [2,3]. The measurement of tissue elasticity is useful for the diagnosis and differentiation of tumors, which are stiffer than normal tissues [4]. The recent introduction of endoscopic ultrasonography elastography (EUS-EG) is a promising imaging technique with a high accuracy for the differential diagnosis of solid pancreatic tumors [5]. Although ovarian cancer is not typical areas within the scope of practice of endosonographers, EUS-EG might be useful to differentiate adnexal masses. We report our experience with EUS-EG in a patient with an adnexal mass.
CASE REPORT
A 74-year-old woman was referred to our hospital with a 1 month history of lower abdominal pain. Her medical history was non-contributory. On admission, abdominal distension was noted during physical examination. Laboratory data showed that serum cancer antigen 125 (CA 125) value was 389 U/mL (reference value, 0 to 35 U/mL). Abdominopelvic CT showed a 6 cm ovoid mass with an enhancing solid portion and a cystic component in the left adnexa and a 1.5 cm heterogeneous enhancing mass in the right adnexa (Fig. 1A). A large amount of ascites was also noted in the abdominal and pelvic cavities. Pelvic dynamic MRI showed a 6 cm heterogenous dark signal intensity in T1-weighted images and a mixed cystic and solid signal intensity mass in T2-weighted images. The solid portion within the cystic mass enhanced strongly after injection of MR contrast (Fig. 1B). PET/CT showed a 5.5 cm oval hypermetabolic mass in the left pelvic cavity (maximum standardized uptake value [SUV], 2.11; average SUV, 2.11) (Fig. 1C).
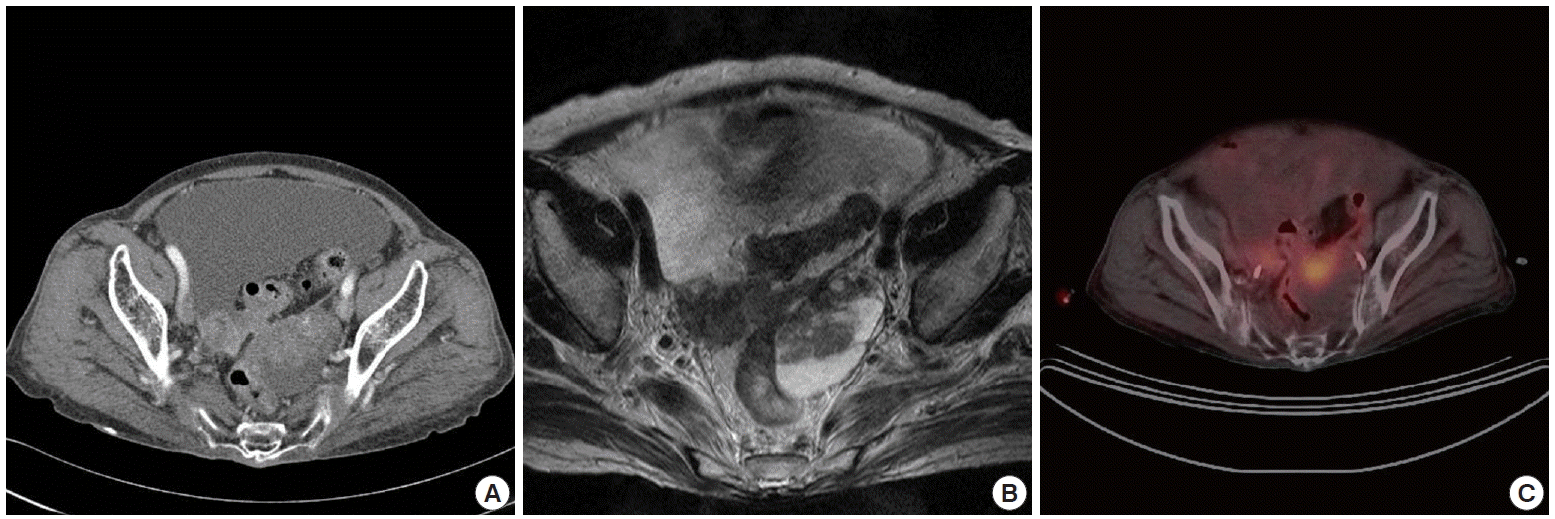
Radiological imaging. (A) Abdomino-pelvic computed tomography (CT) shows a 6 cm ovoid mass with an enhanced solid portion and a cystic component in the left adnexa and 1.5 cm heterogeneous enhanced mass in the right adnexa. (B) Pelvic dynamic magnetic resonance imaging shows a mixed cystic and solid signal intensity mass in T2-weighted images of the left ovary. (C) Positron emission tomography/CT shows an oval hypermetabolic mass in the left pelvic cavity.
She underwent colonoscopy for evaluation of adnexa mass which compressed rectosigmoid colon on abdominopelvic CT. A huge subepithelial lesion was seen at the rectosigmoid colon. EUS was performed to evaluate the characteristics of the subepithelial lesion using an EG-3670 URK electronic radial type ultrasonographic endoscope (Pentax, Tokyo, Japan) and an EUB-7500 ultrasound (Hitachi, Tokyo, Japan). Endoscopic ultrasound showed a left adnexal mass near the rectosigmoid colon (Fig. 2A). We applied EUS-EG to the adnexal mass. Elastograpic imaging of the adnexal mass was characterized by a heterogeneous distribution of blue color, representing stiffness (Fig. 2B). We performed EUS elastography for the quantitative analysis of tissue stiffness in the adnexal masse. Two different areas (A and B) from the region of interest were selected for quantitative elastographic analysis. Area A (blue color) is a representative area of the adnexal mass. Area B refers to a soft (green) adenexal reference area outside the tumor. The quotient B/A (strain ratio) is considered as the measure of the elastographic evaluation. The strain ratio of the adnexal mass was 8.52 (Fig. 2C).

Endoscopic ultrasonography elastographic view. (A) B-mode shows a left adnexal cystic mass near the rectosigmoid colon. (B) Elastographic imaging shows a heterogeneous distribution of blue color. (C) Elastographic strain ratio (quotient B/A) was 8.52.
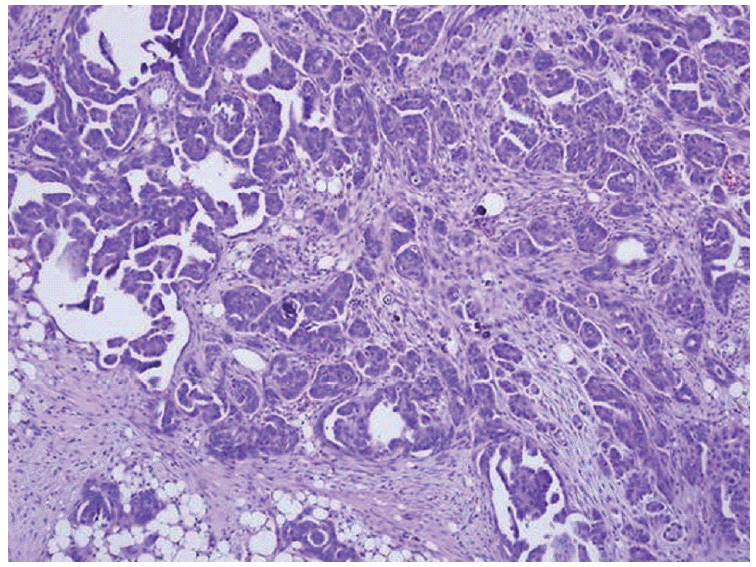
She underwent peritoneal resection, omentectomy, and appendectomy because the ovarian mass adhered densely to the adjacent organs. The omentum and peritoneum were matted by innumerable conglomerated grayish white nodules. They were infiltrated by atypical cuboidal to columnar cells in microcysts and micropapillae with multiple psammoma bodies on a desmoplastic background (Fig. 3). The tumor cells were positive for cytokeratin and CA125 and negative for cytokeratin 20, thyroid transcription factor-1, Caudal-related homeobox 2, and calretinin. The findings were compatible with metastatic papillary adenocarcinoma from the ovary.
DISCUSSION
PET/CT has been reported to be more accurate than pelvic ultrasound, abdominopelvic CT, and pelvic MRI in the differential diagnosis of ovarian cancer, recently [6]. In distinguishing malignant/borderline tumors from benign ovarian masses, the accuracy of PET/CT (92%) was higher than that of pelvic ultrasound (83%) and abdominopelvic CT or pelvic MR imaging (75%). The sensitivity for detection of ovarian cancer using PET is likely dependent on the lesion histology as well as the lesion size. Small lesions, less than 1 cm in size, are likely to be more difficult to detect than larger lesions because of the resolution limitations of current PET systems. Detection of microscopic disease is beyond the resolution of PET/CT systems. Possible causes of false-positive findings on fluorodeoxyglucose (FDG)-PET includes endometrioma, dermoid cyst, cystadenoma, thecoma, schwannoma, follicular cyst, corpus luteum cyst, hydrosalphinx, adnexal abscess, and cholesterol granuloma [7]. Possible causes of false-negative findings includes borderline tumors (mucinous and serous), mucinous adenocarcinoma, serous adenocarcinoma (well-differentiated stage I), small tumors, and hyperglycemia [7]. False positive and false negative findings mean that PET/CT may be inadequate to preclude surgery in women with adenexal masses.
The relevance of EUS in the diagnosis of gynecologic diseases is unknown. EUS can image pelvic pathology in addition to anorectal disease, including endometriosis and ovarian cancer [8,9]. It is difficult to determine the benign or malignant nature of an adnexal mass with EUS alone. EUS-EG is useful for differentiating solid pancreatic masses [5]. Elastographic imaging of a normal pancreas is characterized by a uniform, homogeneous green color distribution (representing intermediate stiffness) throughout the organ, and the reproducibility of the signal is comparatively good. The predominant blue color with either a homogeneous or heterogeneous distribution pattern suggests the diagnosis of a malignant tumor. In this context, we applied EUS-EG to the adnexal mass. In our patient, EUS-EG showed a blue color with a heterogeneous distribution, which suggested malignant tumor. Our patient presented with adnexal mass of less intense FDG uptake (average SUV, 2.11). It appeared that EUS-EG provided complementary information on the biologic behavior of adnexal masses in the setting of residual uncertainty of PET/CT study.
To the best of our knowledge, gastric metastasis of ovarian cancer has been diagnosed in one case using EUS-EG [10]. A gastric submucosal tumor had some features suggesting lipoma because it was hyperechoic and homogeneous in EUS. But, elastography showed increased stiffness of the tissues (blue). The final cytological diagnosis is gastric metastasis from ovarian adenocarcinoma [10]. We also use elastrography to obtain additional information about tissue elastricity. Elastography showed increased stiffness of the adnexal mass (blue), compatible with malignant cancer. These results provide evidence that EUS-EG may be potentially useful to diagnosis of ovarian malignancy.
Previous EUS elastographic study of ovarian cancer was performed by first generation EUS elastography provided only qualitative analysis. But, we performed quantitative analysis not only qualitative analysis of tissue stiffness using second generation elastography (strain ratio, 8.52). The cut-off value of strain ratio for malignant pancreatic tumor and malignant mediastinal lymph node is 6.04 [11] and 7.5 [12]. But, the cut-off value of strain ratio for malignant ovarian tumor is unknown. Futher studies of EUS-EG quantitative analysis will be required for ovarian tumor.
EUS-EG may be useful to differentiate adnexal masses. Future studies will be required to ascertain the diagnostic accuracy of EUS-EG in the differentiation of benign form malignant lesions in patients presenting adnexal masses.