A Case of Complex Restenosis of Aortoiliac Stent Mimicking Downward Stent Migration
Article information
Abstract
We present the case of aortoiliac stent restenosis which was caused by 13 years’ of neointimal progression within and at the edge of the aortoiliac stent at the iliac bifurcation. A 74 year-old man presented with vertigo. We planned 4-vessel cerebral angiography through the right common femoral artery to evaluate his carotid artery but failed due to the catheter jam against the struts of the previously deployed aortoiliac stent. Retrograde sheath angiography through the right femoral artery indicated that the previously implanted stent seemed to have migrated in a downward direction and be embedded in the internal iliac artery. While comparing with the previous angiograms, we found that the implanted stent did not migrate downwardly but was separated from the external iliac artery by newly formed septum of neointimal hyperplasia. We successfully reopened the stenosis using the contralateral approach after widening the struts of the previously deployed T-stents.
INTRODUCTION
Although percutaneous angioplasty had brought many benefits in lower extremities intervention, complications were reported up to 10% of the intervention [1,2]. A relatively common complication after the stent deployment is in-stent restenosis with the incidence of 10% and 50% [3]. Most phenomena occurred within 6 months after the stent placement, but it was often reported in peripheral artery intervention after a longer time period [4,5]. We observed a case of aortoiliac stent restenosis but it was realigned and embedded to the internal iliac artery (IIA) by neointimal hyperplasia 13 years after the deployment which mimicked downward stent migration. So, we introduce this case with the techniques of angioplasty as solution.
CASE REPORT
A 74 year-old man with medical history of diabetes and hypertension was admitted to Wooridul Hospital for vertigo. He underwent goretex bypass surgery from the left common carotid artery to subclavian artery in 1993 due to total obstruction of left subclavian artery. At that time he also had significant stenosis on both common iliac arteries (CIA), so a balloon angioplasty was performed before discharge. In 1997, angiography was performed because intermittent claudication of both his legs indicated over 70% restenosis on both CIA with eccentric 50% narrowing at the infra-renal aorta (Fig. 1A). Thus, we performed balloon angioplasty with stent deployment at these aortoiliac lesions using the T-stenting method (Wallstent; Schneider, Bulach, Switzerland: 12×46 mm at the left CIA; 14× 39 mm at the right CIA to the aorta). Good stent expansion and patency were observed in follow-up angiography in 1999 (Fig. 1B). In 2007, he underwent coronary angiography for chest pain evaluation, and the result indicated total occlusion of the posterior lateral branch of the right coronary artery with the collateral flows coming from the left circumflex artery. He also took a brain magnetic resonance angiography (MRA) for a concomitant dizziness, indicating insignificant stenosis on the left proximal internal carotid artery. However, when he was admitted 2 years after the last examination, follow-up MRA revealed total occlusion of the left internal carotid artery with newly developed right external carotid artery stenosis. We planned 4-vessel cerebral angiography for further evaluation.
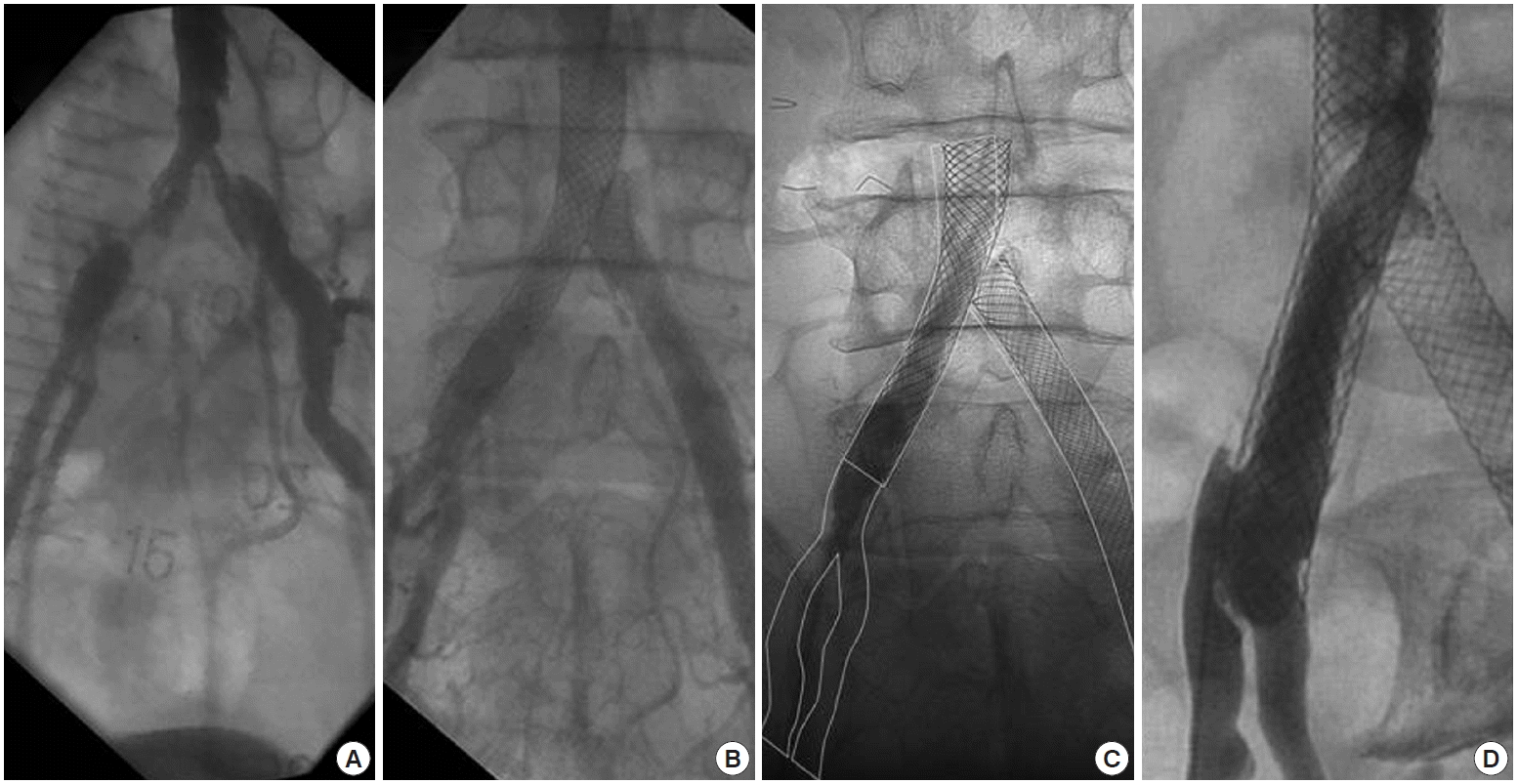
Serial angiographic follow-up of implanted stents during 13 years observation. (A) An angiography taken in 1997 shows over 70% restenosis on previously treated both common iliac arteries with eccentric 50% luminal narrowing above the aortic bifurcation site. (B) An angiography taken in 1999 shows good patency and expansion of previously deployed stents by the T-stenting method. (C) An angiography taken at this presentation shows the previously deployed stents are positioned without migration comparing to the 1999’s angiography. The lines indicate the outlines of stents and vessels of 1999’s angiography which moved to the current angiography for comparing the stents position. (D) An angiography at the right anterior oblique projection indicates that the right aortoiliac stent is embedded in the internal iliac artery by neointima which was accompanied with eccentric 70% luminal narrowing at the ostium of the right external iliac artery.
After puncturing the right common femoral artery (CFA), a 0.889 mm guidewire (curved tip hydrophilic wire; Terumo, Tokyo, Japan) with a 5 Fr multi-purpose catheter (MP catheter, Terumo) was used to pass through the previous stent. However, the catheter stuck at the distal two third portion of the implanted stent. So, sheath angiography was performed at the left anterior oblique projection, which revealed eccentric 70% luminal narrowing at the ostium of the right external iliac artery (EIA) (Fig. 1D). Furthermore, the stent which was previously implanted from the aorta to the right CIA seemed to have migrated downwardly and be embedded in the IIA. We found that the inserted guidewire passed between the anterior struts of the previously implanted stent. We could not understand this delayed downward stent migration because the filling defects over the stent struts indicated diffuse neointimal growth (Fig. 1D) and the previously deployed stent was well expanded and flared up at the aortic side on angiography. So, we compared the current angiography with the one taken in 1999 and found that the previously deployed stents were positioned in the exactly same places without migration (Fig. 1C). The distal edge of the stent was directed toward the IIA, and neointimal proliferation made the new iliac bifurcation above the true iliac bifurcation (Fig. 2). Although the patient did not complain of any symptoms on his legs, we decided to treat this lesion because his atherosclerotic lesions of carotid and iliac arteries became progressively worse even during the optimal medical treatment with glucose control. Furthermore, it required a vascular access to prepare further treatment of carotid or coronary intervention.

Schematic pictures of implanted aortoiliac stent at the right iliac artery. (A) A schematic picture represents initial position of distal edge of aortoiliac stent. (B) A schematic picture represents changed position of distal edge of aortoiliac stent at this presentation which was realigned toward the internal iliac artery and embedded by neointima. Dotted line: newly formed neointima. EIA, external iliac artery; IIA, internal iliac artery.
First, we tried to make wire passage to the EIA through the ipsilateral retrograde approach rather than contralateral side approach to preserve the deployed stents by the T-stenting method. To obtain catheter entrance, balloon angioplasty (4×40 mm; Powerflex, Cordis, Miami, FL, USA) was done through the wire entry site between the struts followed by the insertion of a 5 Fr Omni flush catheter (AngioDynamics, Queensbury, NY, USA), a 0.889 mm wire (curved tip, Terumo) was attempted to pass the neointimal septum through the distal stent lumen (Fig. 3A), and it seemed to have penetrated the septum. We inserted a snare and successfully pulled the wire out of the sheath. After pulling the Omni ca theter out of the stent, the curved tip of the wire was slowly drawn until the opposite tip of the wire jumped out to the aortic side, and then the wire was carefully pushed to the proximal aortic lumen of the stent. With this method, we thought that the opposite site of the wire was headed toward the aorta while keeping the wire still located between the penetrated neointimal spaces. However, the follow up angiography of the left anterior oblique projection revealed that the wire was still located between the stent struts. The wire from the Omni catheter did not pass through the neointima of the distal stent lumen but through between the struts from the first. Despite several attempts, we failed to cross the wire into the EIA due to the thickened neointima.
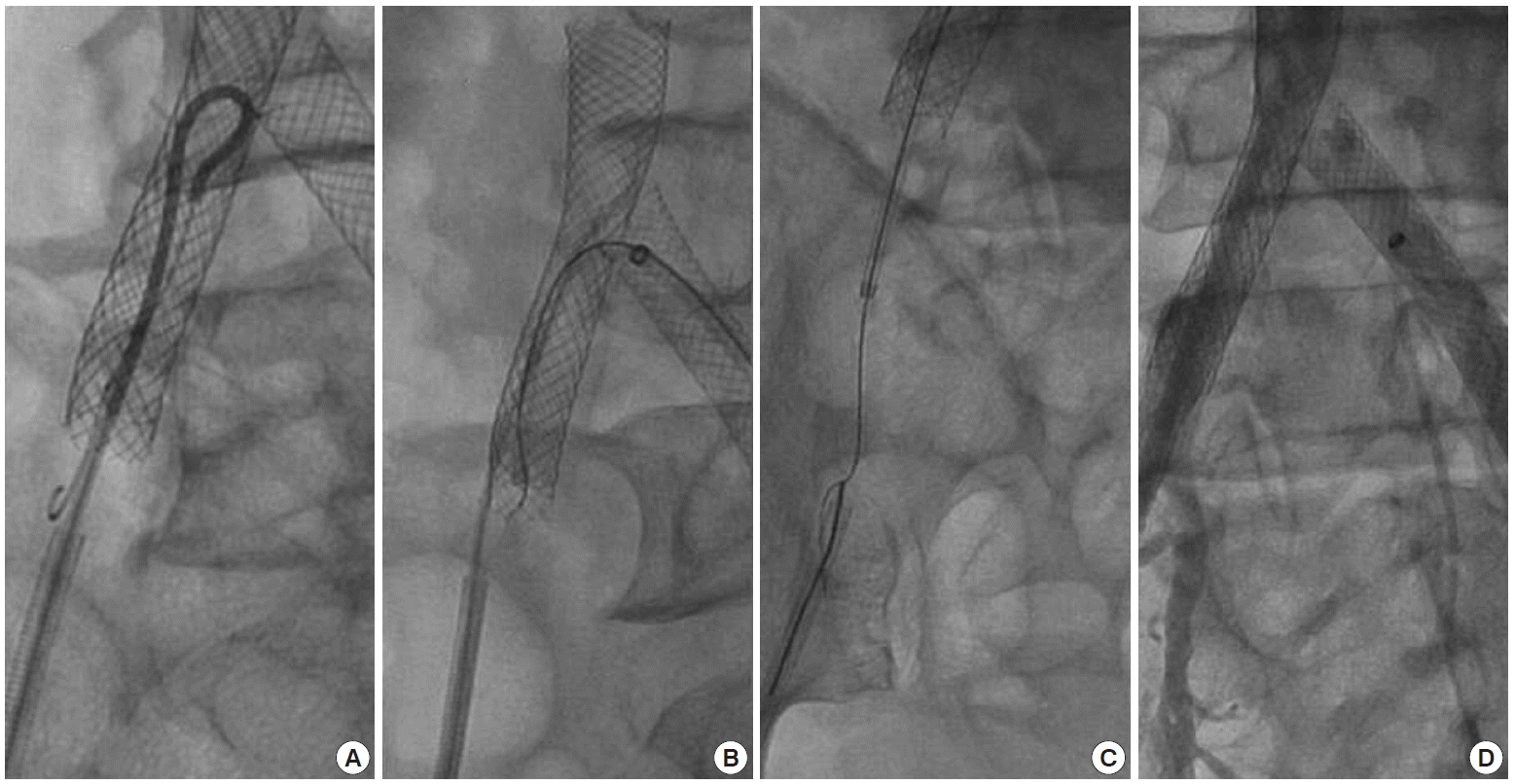
Applied techniques to access to the external iliac artery. (A) A 0.889 mm wire from the 5 Fr Omni flush catheter is attempted to pass the neointima through the distal stent lumen. (B) A 0.3556 mm guidewire with a microcatheter over the glide catheter is inserted through Balkin sheath from the left common femoral artery. (C) A guidewire is passed into the right external iliac artery through the distal stent lumen. A guidewire caught by the snare is pulled out of the right femoral sheath. (D) A final angiography through the right femoral sheath reveals a self expandable stent is well deployed overlapping with previous right aortoiliac stent.
We then attempted another procedure after puncturing the left CFA. A 0.035 inch wire (curved tip, Terumo) was passed between the struts of the proximal right side of the wall stent at the T-stenting site. To secure enough space for catheter entering, a balloon was inflated to widen the gaps between struts. A Balkin sheath (7 Fr; Cook, Bloomington, IN, USA) was inserted through the left CFA, then a 0.014 enhanced force guidewire (Conquest Pro 9 g; Asahi Intec Co., Aichi, Japan) with a microcatheter (Progreat; Terumo) over the glide catheter (4 Fr, Terumo) was inserted through the left CFA (Fig. 3B). Because proliferated neointima of the distal edge of the stent was thickened between the EIA and IIA, this approach was also performed with difficulty. However, after many repetitions, the wire finally passed into the right EIA through the distal stent lumen. After catching the wire with the snare (Fig. 3C), gradually larger sized balloon angioplasties (from 4×40 mm [Powerflex; Cordis] to 7×40 mm UTD [Boston Scientific Co., Natick, MA, USA]) were performed at the iliac bifurcated site, and then a self-expandable stent (9× 60 mm, Smart; Cordis) was deployed overlapping with previous stent as the final step (Fig. 3D).
DISCUSSION
A stent deployed to the CIA 13 years ago was embedded in the IIA, thanks to neointimal growth. We first considered this case as delayed downward stent migration. If the stent was migrated into the IIA, the reopening procedure at the distal edge of the stent was rarely possible because of the risk of vessel rupture. However, as far as we know, there was only one case report of delayed stent migration in intervention of aortoiliac arteries [6]. Even in that report, the previously deployed iliac stent migrated in an upward direction. If the stent expanded well, the downward migration could not have possibly taken place in aortoiliac stenting because the aortic dimension is almost always larger than that of the iliac artery. Importantly, the previously deployed stent in our case expanded well and flared up at both sides of the edges on the angiography. So, we used side to side comparison then, finally considered this stent embedding was not a result of downward stent migration but of neointimal progression above the iliac bifurcation.
Although a stent placement has been shown potential advantages, including better technical success, resistance to elastic recoil, and patency in iliac artery intervention [7], neointimal hyperplasia is known as more stimulative than balloon angioplasty [8,9]. Moreover, the restenosis process of a peripheral artery does not appear to stop at 6 months, but continues for a longer time, even up to several years, after the intervention [4,5]. Thus, very late restenosis by neointimal proliferation is occasionally reported in a case with peripheral artery intervention. One possible explanation for our case is the distal edge of the stent was more firmly attached to the lateral side of the IIA than the EIA at the time of deployment, then neointimal proliferation began to start at the distal edge of the bifurcation site and made a separation between the EIA and IIA. Although the follow up angiography 2 years after the stent implantation indicated a good expansion of the distal portion of the stent at the right CIA, there might have been the malapposed portion at the CIA due to aneurysmal dilatation which was observed in the angiography taken in 1997 (Fig. 1A).
We described a rare case of aortoiliac stent restenosis at the iliac bifurcation and introduced the several techniques to this complicated restenosis.