ABSTRACTObjectiveBone marrow (BM) examinations are performed to evaluate hematological abnormalities. Focusing on patients with cytopenia, we aimed to determine the circumstances under which a BM examination can assist in the diagnosis of hematologic diseases.
MethodsThe medical records of 738 patients who underwent BM examination from March 2011 to March 2019 at Soonchunhyang University Seoul Hospital were reviewed. In total, 234 patients underwent a BM examination to identify the cause of cytopenia. Excluded from the analysis were BM examinations performed to diagnose specific diseases and evaluate disease status.
ResultsResults suggesting suboptimal outcome (n=6) or BM invasion of solid tumors (n=13) were excluded. Immune thrombocytopenic purpura patients (n=52) with normal BM examination results were also excluded. One hundred sixty-three patients who underwent BM examination to determine the cause of cytopenia were included in the analysis. A comparison of non-specific results (n=56) to those pointing to an underlying hematologic disease (n=107) showed that patients with severe neutropenia or severe thrombocytopenia were more likely to be diagnosed with a hematologic disease. Specifically, as the number of severe cytopenias increased, the likelihood of a hematologic disease diagnosis was significantly augmented. Patients with end-stage renal disease, autoimmune disease, or liver cirrhosis were more likely to receive non-specific results.
INTRODUCTIONBone marrow (BM) examination is the analysis of a BM tissue sample to evaluate the structure and the different types of blood cells of the BM. It is performed in conjunction with a complete blood count (CBC), a peripheral blood smear, and sometimes additional testing on the BM, to provide information about BM integrity and its capacity for blood cell production, including red blood cells (RBC), white blood cells (WBC) and platelets. BM examination allows for the diagnosis and assessment of various conditions, such as primary hematologic and metastatic neoplasms as well as nonmalignant disorders. Even though it is considered a safe procedure, the British Journal of Hematology reported complications in 26 cases out of 54,890 BM examinations (0.047%). The most frequent and serious adverse event was hemorrhage, reported in 14 patients [1]. Also, BM examination is an invasive procedure associated with intense pain and a considerable amount of time spent in the hospital to ensure hemostasis. For these reasons, clinicians often hesitate to order a BM examination.
Peripheral blood cytopenias are common in adults, especially in the elderly, and can either be inconsequential or a sign of serious disease. A specific cause of cytopenia can usually be identified by a careful examination of the medical history, a targeted physical examination, and appropriate laboratory and imaging tests, supplemented, under certain circumstances, by a BM examination. However, in some patients, diagnostic uncertainty persists and the cause underlying cytopenia may remain unknown, even after thorough evaluation [2]. In this study, we reviewed the cases and results of BM examination that took place at the Soonchunhyang University Seoul Hospital over an 8-year period. Hence, we aimed to determine whether patients with cytopenias of undiagnosed cause should actively consider BM examination to facilitate differential diagnosis of a hematologic disease.
MATERAIALS AND METHODS1. SubjectThis retrospective study was carried out at Soonchunhyang University Seoul Hospital considering the medical records of 738 patients who underwent BM examination from March 2011 to March 2019. A total of 1,124 BM examinations were performed and the analysis was carried out on initial BM examinations. Ninety-five patients who underwent BM examination for the diagnosis of plasma cell disorders and 210 patients who underwent the procedure for the staging workup of lymphoma were excluded. Sixty-one patients who were diagnosed at other hospitals or Soonchunhyang University Hospital before March 2011 were excluded. Furthermore, seven patients examined to detect BM involvement in hemochromatosis, mastocytosis, and Langerhans cell histiocytosis or for clinical trial registration and stem cell collection were excluded. Out of 365 patients with abnormal CBC, 39 patients who were diagnosed with acute leukemia in more than 20% of immature cells of the peripheral blood were excluded.
2. Definition of complete blood count abnormalityThe normal range for a WBC count in adults is 4,400 to 11,000 cells/μL. Neutrophilia refers to an increase in the number of peripheral blood neutrophils by at least two standard deviations. In most clinical laboratories, this corresponds to >7,700 neutrophils/μL in adults, typically observed in patients with WBC >11,000 cells/μL. Neutropenia is usually defined as an absolute neutrophil count (ANC) of <1,500 cells/μL in adults [2]. Furthermore, severe neutropenia is defined as an ANC of <500 cells/μL [3]. Polycythemia (erythrocytosis) refers to an increased concentration of hemoglobin (>16.5 g/dL in men or >16.0 g/dL in women) and/or hematocrit (>49% in men or >48% in women) in peripheral blood [4]. World Health Organization criteria for anemia in men and women are <13 g/dL and <12 g/dL, respectively. In general, different guidelines recommend the use of a restrictive transfusion threshold within 7 to 8 g/dL [5–7]. In this study, severe anemia is defined as a hemoglobin <7 g/dL. Thrombocytosis is defined as a platelet count of >450,000 cells/μL in adults. Thrombocytopenia is defined as a platelet count below the lower normal threshold, which is<150,000 cells/μL for adults. Degrees of thrombocytopenia can be further subdivided into mild (platelet count 100,000 to 150,000 cells/μL), moderate (50,000 to 99,000 cells/μL), and severe (<50,000 cells/μL) [8].
When WBC, RBC, and platelet counts increase, a BM test is performed to diagnose chronic myeloid leukemia or other myeloid proliferative neoplasm. Usually, leukocytosis is defined as more than 11,000 WBC/μL, but in this study, only patients with more than 15,000 WBC/μL were included. Erythrocytosis and thrombocytosis were also based on the criteria outlined above. Eighty patients underwent a BM examination to discern the cause of a CBC increase. Twelve patients with suspected hypereosinophilic syndrome were also excluded. Finally, we examined 234 cases of BM examination performed to identify the cause of cytopenia, except when used for the diagnosis of a specific disease or the evaluation of the disease status (Fig. 1).
Thus, the medical records of a population of 234 patients with cytopenia were reviews. Specifically, the analysis considered information about the age, sex, comorbidity, CBC, lactate dehydrogenase, and BM report of the population. The protocol in use was approved by the Institutional Review Board of Soonchunhyang University Seoul Hospital (IRB no., 2019-08-013). The requirement for informed consent from individual patients was omitted because of the retrospective design of this study. The R ver. 3.6 software (The R Foundation for Statistical Computing, Vienna, Austria) was used for all statistical analyses.
RESULTSBaseline characteristics of patients with cytopenia are shown in Table 1. A comparison of two groups of patients is given in Table 2; one group received non-specific BM results and another received a hematologic disease diagnosis. Cases of suboptimal outcome (n=6) and BM invasion of solid tumors (n=13) were excluded. BM examinations of immune thrombocytopenic purpura (ITP) patients usually show normal cellularity and normal or increased numbers of megakaryocytes, excluding the possibility of BM-induced thrombocytopenia. Thus, ITP patients (n=52) were excluded. One hundred sixty-three patients who underwent BM examination to determine the cause of cytopenia were analyzed (Table 2). Cytopenia was significantly more severe in the hematologic disease group than in the non-specific group (Fig. 2). In 75 cases of pancytopenia, 12 cases of aplastic anemia, 14 cases of acute leukemia, and 27 cases of myelodysplastic syndrome (MDS) were confirmed, while 12 cases were nonspecific. In non-specific cases, cytopenia was recovered in five cases due to infection, one case was caused by Behçet’s disease, and one case was caused by chemotherapy for solid cancer. Three patients were eventually diagnosed with acute promyelocytic leukemia, peripheral T-cell lymphoma, and hepatitis C viral infection, respectively, while two patients remained undiagnosed.
In cases of severe neutropenia or severe thrombocytopenia, hematologic diseases were more likely to be diagnosed. Especially, as the number of severe cytopenia findings in a patient increased, the possibility of reaching a hematologic disease diagnosis increased as well. Furthermore, patients with end-stage renal disease (ESRD), autoimmune diseases or liver cirrhosis were more likely to receive nonspecific BM examination results. In the non-specific group, patients with cytopenia caused by infection (n=10) or megaloblastic anemia (n=6) were found to consistently recover from cytopenia. Among those who received a non-specific BM examination result were eight patients with ESRD, three patients with chronic kidney disease (CKD) without dialysis, three patients with autoimmune disease, and one patient with liver cirrhosis. The patients who received a hematologic disease diagnosis were three patients who were administered chemotherapy or radiotherapy as cancer treatment and two patients who underwent surgery (kidney transplantation, Whipple operation). Additionally, four patients were diagnosed with other diseases and the remaining 15 patients did not obtain an explanation for the underlying cause of cytopenia at the time of BM examination. The characteristics of the patients with unexplained cytopenia are shown in Table 3. Five of them were diagnosed with other diseases (acute myeloid leukemia [n=1], MDS [n=1], hepatitis C viral infection [n=2], and Sjögren’s syndrome [n=1]) during a follow-up period.
DISCUSSIONBM examinations serve to confirm the diagnosis of a disease or to determine a disease stage. However, in patients with cytopenia, clinicians are reluctant to order a BM examination, due to the invasive nature of the procedure. Also, many studies have explored the sensation of pain caused by a BM examination [9]. Even though the present institution uses pethidine for pain control, patients still occasionally complain of severe pain and other adverse events, such as bleeding and infection. In this study, no severe complications were reported for the 1,124 BM examinations.
Cytopenia is a clinically significant cause of concern and seeking a diagnosis for the underlying cause is encouraged. Conditions that manifest as cytopenia include liver disease, renal disease and autoimmune diseases or drug consumption [10,11]. In the present study, CKD was identified as the cause of cytopenia in 11 patients, including eight patients with ESRD. When CKD is prolonged, erythropoietin (EPO) secretion by the kidney decreases and toxic metabolic waste accumulates, causing hematological changes, including a decrease in RBC and platelet counts. Specifically, the exact pattern underlying platelet count changes in patients with CKD is ambiguous, but several studies support that platelet number decreases [12]. Furthermore, two out of three ESRD patients and a hematologic disease underlying cytopenia were diagnosed with pure red cell dysplasia (PRCA), which is known to be associated with EPO use in ESRD [13]. Therefore, PRCA caused by EPO may be related to ESRD pathogenesis.
Cytopenia in 76-year-old woman with severe neutropenia, severe anemia and a non-specific BM examination result was attributed to azathioprine administration for the treatment of microscopic polyarthritis. Azathioprine is a thiopurine prodrug commonly used as an immunosuppressive agent in the treatment of autoimmune and hematologic diseases. Thiopurine S-methyltransferase (TPMT) metabolizes azathioprine to its inactive form. Variant TPMT alleles cause slow azathioprine break-down and are associated with azathioprine-induced leukopenia [14]. Also, infection can cause transient cytopenia, as observed in 10 patients whose cytopenia remained undiagnosed by the BM exam and improved when the infection resolved. Therefore, it is essential that a cytopenia finding is followed-up with investigation of its cause.
Especially in the case of severe cytopenia, various examinations, including BM examination, are required to confirm the cause. When severe cytopenia was detected in multiple cell lineages it was associated with hematologic disease. All patients with severe cytopenia in three lineages were diagnosed with a hematologic disease. Six patients with severe cytopenia in two lineages received a nonspecific BM examination result. Furthermore, four patients with infection-induced cytopenia, one patient diagnosed with megaloblastic anemia and one case of azathioprine treatment recovered from cytopenia. Finally, 20 patients presented with severe cytopenia in one lineage (infection [n=2], megaloblastic anemia [n=3], ESRD [n=4], autoimmune disease [n=2), and unknown cause [n=5]). Thus, patients with severe cytopenia in several lineages can benefit more from a BM examination.
The diagnostic criteria for ITP do not require a BM examination. BM examinations were previously considered a routine diagnostic test in ITP patients, who were >60 years of age, to exclude the possibility of a MDS [15,16]; however, this practice is no longer recommended as long-term monitoring of older adults with ITP has not revealed an increased incidence of MDS [17,18]. Out of 28 patients with isolated thrombocytopenia, 25 patients were diagnosed with ITP after BM examination. The remaining three with isolated thrombocytopenia were not diagnosed with a hematologic disease (infection [n=1], cholangiocarcinoma with concurrent chemoradiation therapy [n=1], and persistent pseudothrombocytopenia [n=1]). As these 12 patients were aged >60, it can be concluded that a BM examination is not necessary in ITP diagnosis, regardless of patient age.
The main limitation of this retrospective study relates to the small sample size acquired from a single center. Additionally, no additional tests, such as Next Generation Sequencing, were used to follow-up non-specific results, except of chromosome analysis. Also, in many occasions sufficient long-term monitoring was not provided. Nevertheless, it must be noted that checking for indicators of BM examination necessity in clinical cases of cytopenia is meaningful.
In conclusion, the course of action for patients with cytopenia should first include the identification of underlying diseases and drugs. In cases of severe cytopenia in more than one cell lineage, BM examination should be actively considered to explore the presence of a hematologic disease.
Fig. 1Classification of patients who underwent bone marrow (BM) examinations. CBC, complete blood cell count; PB, peripheral blood. 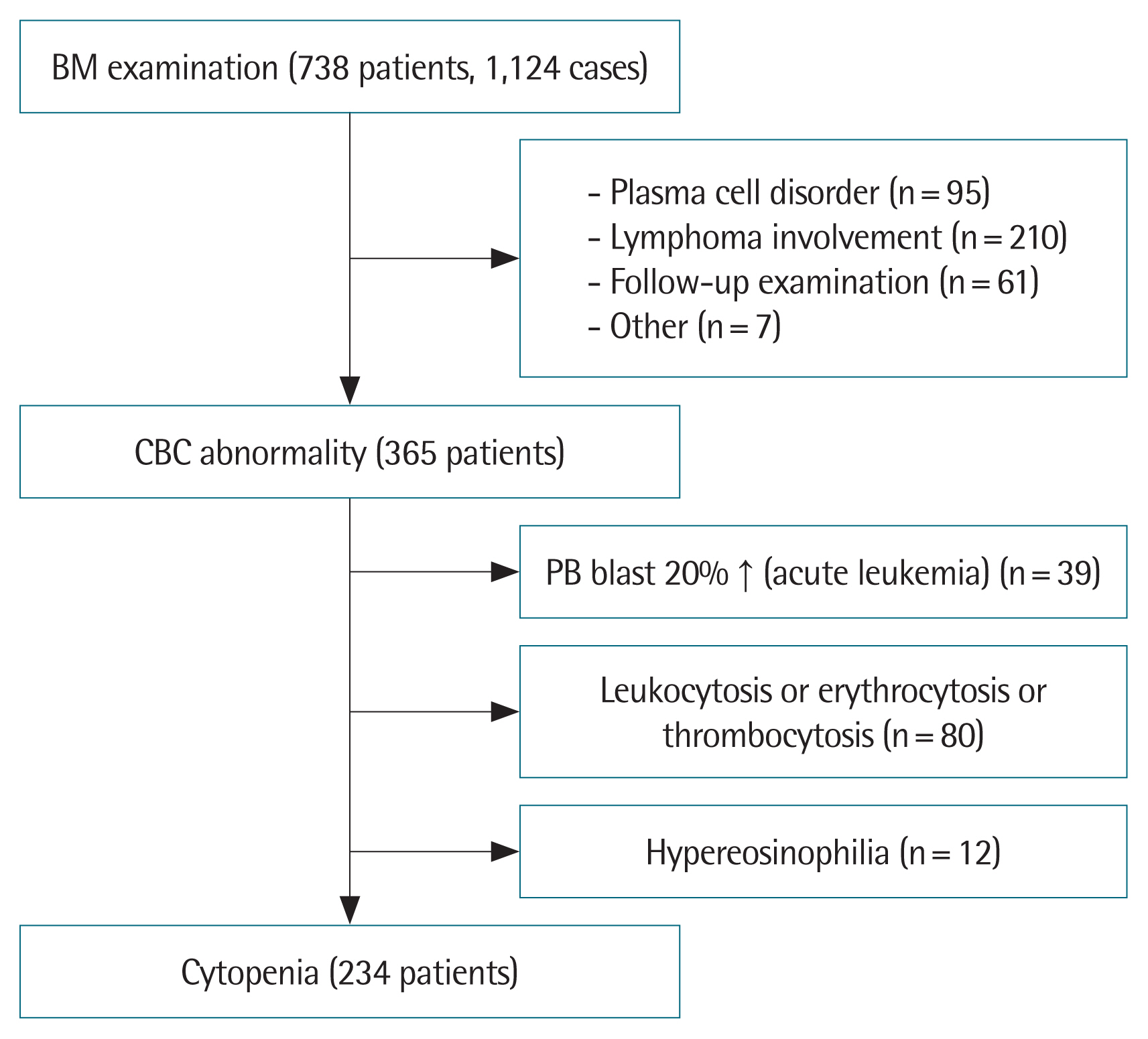
Fig. 2Comparison of group of non-specific bone marrow examination results (group A, black color) and hematologic disease group (group B, white color). (A) White blood cell (WBC): P=0.036. (B) Absolute neutrophil count (ANC): P<0.001. (C) Hemoglobin: P=0.006. (D) Platelet: P=0.001. 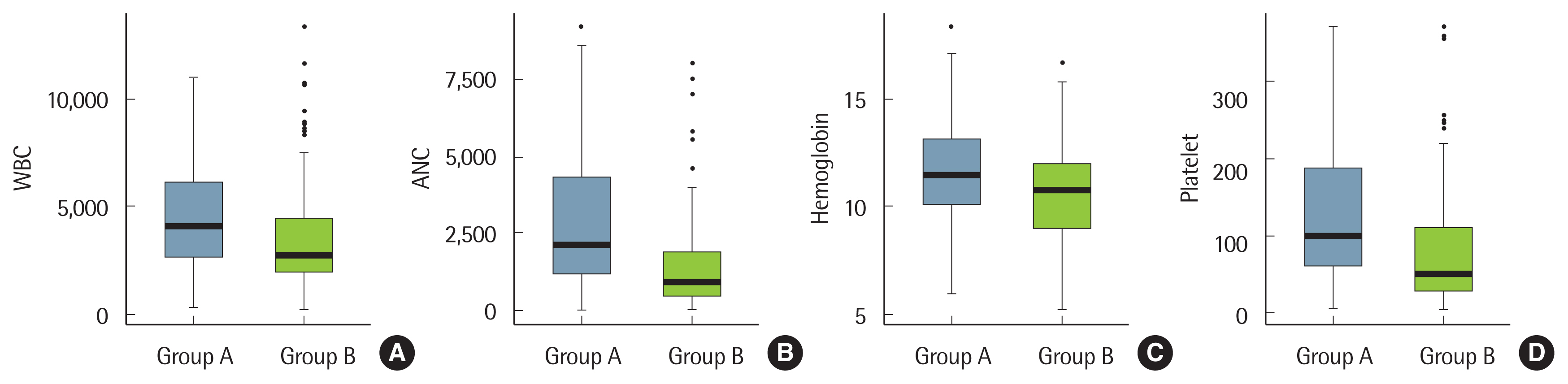
Table 1Baseline characteristics of patients with cytopenia
Table 2Comparison of hematologic disease group and non-specific group
Table 3Patients with unexplained cytopenia
CBC, complete blood count; WBC, white blood cell; ANC, absolute neutrophil count; Hgb, hemoglobin; PLT, platelet; LDH, lactate dehydrogenase; FU, follow-up; F, female; M, male; ICUS, idiopathic cytopenia undetermined significance; R/O, rule out; MDS, myelodysplastic syndrome; EH, erythroid hyperplasia; HCV, hepatitis C viral infection. REFERENCES2. Valent P. Low blood counts: immune mediated, idiopathic, or myelodysplasia. Hematology Am Soc Hematol Educ Program 2012;2012: 485-91.
4. Barbui T, Thiele J, Gisslinger H, Kvasnicka HM, Vannucchi AM, Guglielmelli P, et al. The 2016 WHO classification and diagnostic criteria for myeloproliferative neoplasms: document summary and in-depth discussion. Blood Cancer J 2018;8: 15.
5. Carson JL, Terrin ML, Noveck H, Sanders DW, Chaitman BR, Rhoads GG, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med 2011;365: 2453-62.
6. Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med 1999;340: 409-17.
7. Villanueva C, Colomo A, Bosch A, Concepcion M, Hernandez-Gea V, Aracil C, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med 2013;368: 11-21.
8. Williamson DR, Albert M, Heels-Ansdell D, Arnold DM, Lauzier F, Zarychanski R, et al. Thrombocytopenia in critically ill patients receiving thromboprophylaxis: frequency, risk factors, and outcomes. Chest 2013;144: 1207-15.
9. Zahid MF. Methods of reducing pain during bone marrow biopsy: a narrative review. Ann Palliat Med 2015;4: 184-93.
10. Dorgalaleh A, Mahmudi M, Tabibian S, Khatib ZK, Tamaddon GH, Moghaddam ES, et al. Anemia and thrombocytopenia in acute and chronic renal failure. Int J Hematol Oncol Stem Cell Res 2013;7: 34-9.
12. Gafter U, Bessler H, Malachi T, Zevin D, Djaldetti M, Levi J. Platelet count and thrombopoietic activity in patients with chronic renal failure. Nephron 1987;45: 207-10.
13. Rahbar M, Chitsazian Z, Abdoli F, Moeini Taba SM, Akbari H. Pure red cell aplasia due to antibody against erythropoietin in hemodialysis patients. J Nephropathol 2017;6: 25-9.
14. Gisbert JP, Nino P, Rodrigo L, Cara C, Guijarro LG. Thiopurine methyltransferase (TPMT) activity and adverse effects of azathioprine in inflammatory bowel disease: long-term follow-up study of 394 patients. Am J Gastroenterol 2006;101: 2769-76.
15. George JN, Woolf SH, Raskob GE, Wasser JS, Aledort LM, Ballem PJ, et al. Idiopathic thrombocytopenic purpura: a practice guideline developed by explicit methods for the American Society of Hematology. Blood 1996;88: 3-40.
16. British Committee for Standards in Haematology General Haematology Task Force. Guidelines for the investigation and management of idiopathic thrombocytopenic purpura in adults, children and in pregnancy. Br J Haematol 2003;120: 574-96.
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||