INTRODUCTION
The incidence of synchronous double primary lung cancer (SPLC) varies widely from 1% to 20% according to the reported clinical series [1,2]. This wide range of results has arisen from vague differential diagnoses between intrapulmonary metastasis (IPM) and SPLC, especially in cases with the same histology in both tumors. Because IPM has shown a poor prognosis and has not been a principal indication of surgical resection, SPLC must be differentiated from IPM from the thoracic surgeons’ perspective. The criteria proposed by Martini and Melamed [3] in 1975 are still being used for the diagnosis of SPLC. In those criteria, tumors which arose from different lobes, if tumors had no distant metastasis and no mediastinal lymph node metastasis, were diagnosed as SPLC even though the same histologies were shown for each lobe. However, it is possible enough to include IPM in those criteria. Moreover, multiple ground-glass opacity (GGO) featured adenocarcinoma or bronchioloalveolar carcinoma (BAC) are commonly found as a result of the widespread use of computed tomography (CT) screening, and such cases are considered to be multiple SPLC. BAC is the former term that presents as one of adenocarcinoma in situ, minimally invasive adenocarcinoma, and some type of invasive adenocarcinoma [4]. Although accurate mechanisms for the consequences of the theory of adenocarcinoma and BAC are not fully understood, it is reasonable that these cell types were considered to be the same histology and multiple GGO featured lung cancer was not considered to be SPLC due to unbelievable long-term survival. Many methods have been developed to reveal the origin of cancer in order to differentiate SPLC from IPM. Some have shown that a high frequency of somatic mutations in the concurrent detection of p53 and epidermal growth factor receptor (EGFR) resulted in a high discrimination rate for tumor clonality of SPLC [5]. In order to minimize the risk of including metastatic secondary nodules that might influence the understanding of outcomes related to SPLC, there is a need for strict criteria for the definition of SPLC, such as the exclusion of the adenocarcinoma featuring multiple GGOs and of cases with the same histology for each tumor based on immunohistochemical (IHC) staining.
Therefore, this study aimed to evaluate the prognosis of strictly selected SPLC.
MATERIALS AND METHODS
1. Patients
The records of patients who underwent complete resection for more than two non-small cell lung cancers (NSCLCs) between January 2006 and December 2016 were reviewed. The inclusion criteria were as follows: first, patients with different histologic types in each tumor regardless of nodal status or locations: second, patients with the same histology in each tumor, but without nodal metastasis or without the same lobe. Exclusion criteria were as follows: first, patients with multifocal GGO featured adenocarcinoma: second, patients who underwent preoperative chemotherapy or chemoradiotherapy: third, patients with the same pattern of IHC staining in each tumor with the same histology: fourth, patients who had a history of prior lung cancer.
2. Treatment
The extent and the time intervals of surgical resection depended on the location and number of the tumors, pulmonary function, and preference of the surgeon. An ipsilateral mediastinal lymph node dissection was also performed. According to the pathologic stage, patients with nodal metastasis were underwent adjuvant chemotherapy or chemoradiation therapy; however, synchronous primary lung cancer itself did not indicate adjuvant therapy.
3. Follow-up
Follow-up consisted of chest CT and tumor marker analysis every 4 months for the first 2 years. Since then, we checked chest CT and tumor marker analysis every 6 months. Positron emission tomography-computed tomography (PET-CT) was performed when recurrence was suspected. Local and distant recurrence were identified using regular chest CT and PET-CT and invasive procedures, including bronchoscopy and endobronchial ultrasound (EBUS). Local recurrence was defined as a recurrence of a tumor at the resection margin, ipsilateral hilum, or the mediastinum.
4. Data collection
Each tumor size measured from the pathologic specimen, the maximum standardized uptake value (mSUV) of each tumor on PET/CT and each staging based on the American Joint Committee for Cancer Staging Manuals eighth edition were evaluated. The IHC status was interpreted on the pathologic reports, which were reviewed by a pathologist.
5. Statistical analysis
Continuous data are presented as means and categorical data are presented as percentages. Fisher’s exact test or the Pearson’s chi-square test was used to assessing categorical data and the Wilcoxon rank sum was used for continuous data. The actuarial survival of patients was estimated by Kaplan-Meier analysis, with P-values calculated by log-rank statistics. Death from any cause was used to determine the overall survival, and only cancer-related deaths were included in the cancer-specific mortality. Recurrence of cancer was used to determine disease-free survival. Statistical analyses were performed using IBM SPSS ver. 20.0 software (IBM Corp., Armonk, NY, USA). All P-values less than 0.05 were considered to be statistically significant.
RESULTS
During the study period, 20 patients who underwent curative resection of NSCLCs were found to meet our criteria. They consisted of 18 males (90.0%) and two females (10.0%) with a mean age of 67.0 years (range, 48–83 years). All the patients had two tumors. The median size of the larger tumor was 2.9 cm (range, 1.2–8.0 cm) and that of the smaller tumor was 1.4 cm (range, 0.5–3.2 cm). The median value of the higher of the mSUV of the tumors was 5.4 (range, 1.7–14.9) and that of the lower mSUV was 1.9 (range, 0–5.4). The tumors were located in the same lobe in four patients, ipsilateral different lobes in eight patients, and contralateral lobes in eight patients. The four patients whose tumors were located in the same lobe had a different histologic type and no nodal metastasis. The most common type of surgical resection was pulmonary resection more than lobectomy (i.e., lobectomy or bilobectomy or sleeve lobectomy) with wedge resection (7 of 20, 35%) followed by pulmonary resection more than lobectomy for both lesions (5 of 20, 25%). Of the three patients who were treated with sublobar resection+non-operation, photodynamic therapy was performed for an endobronchial lesion in three patients to avoid a pneumonectomy (Table 1).
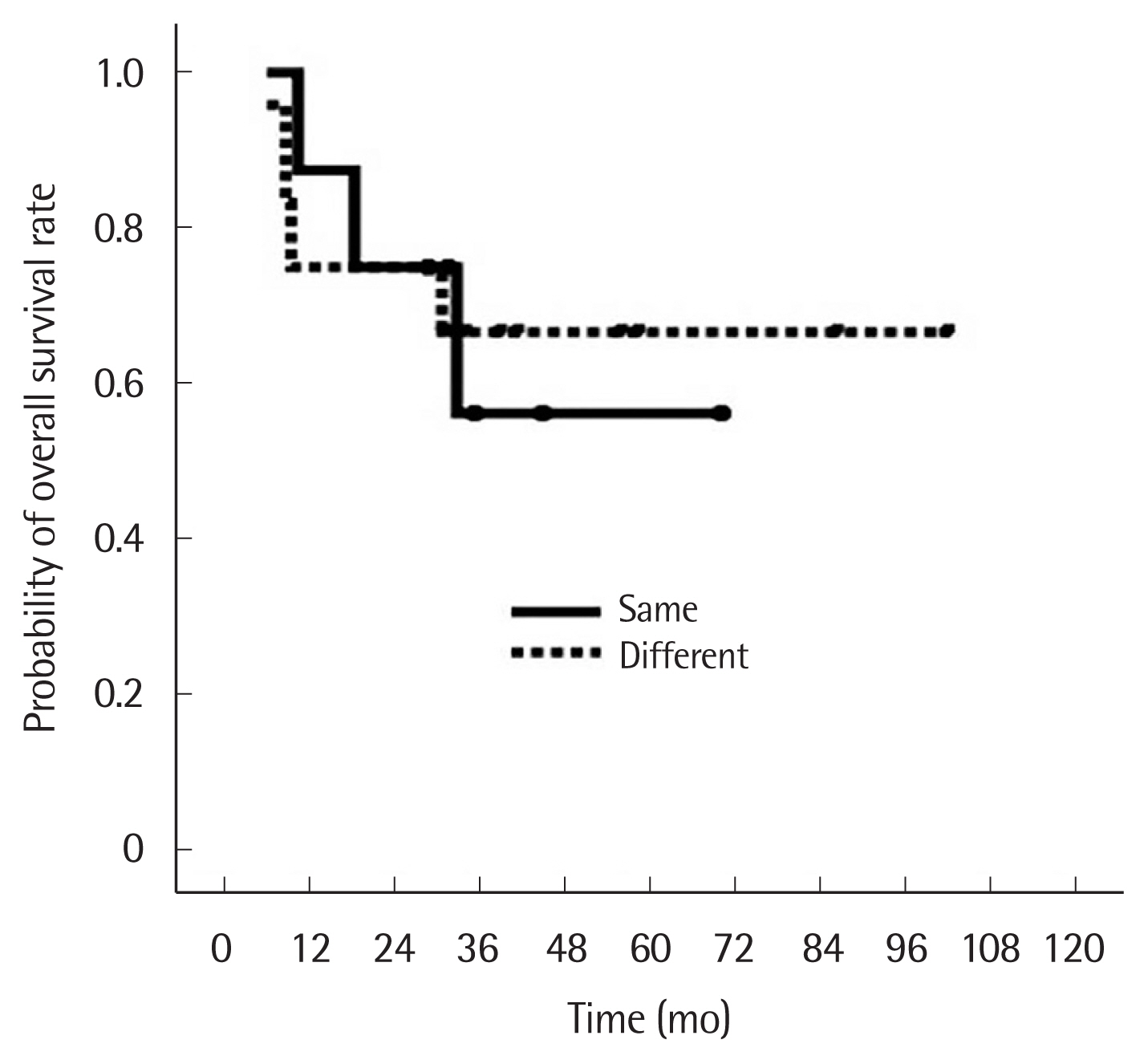
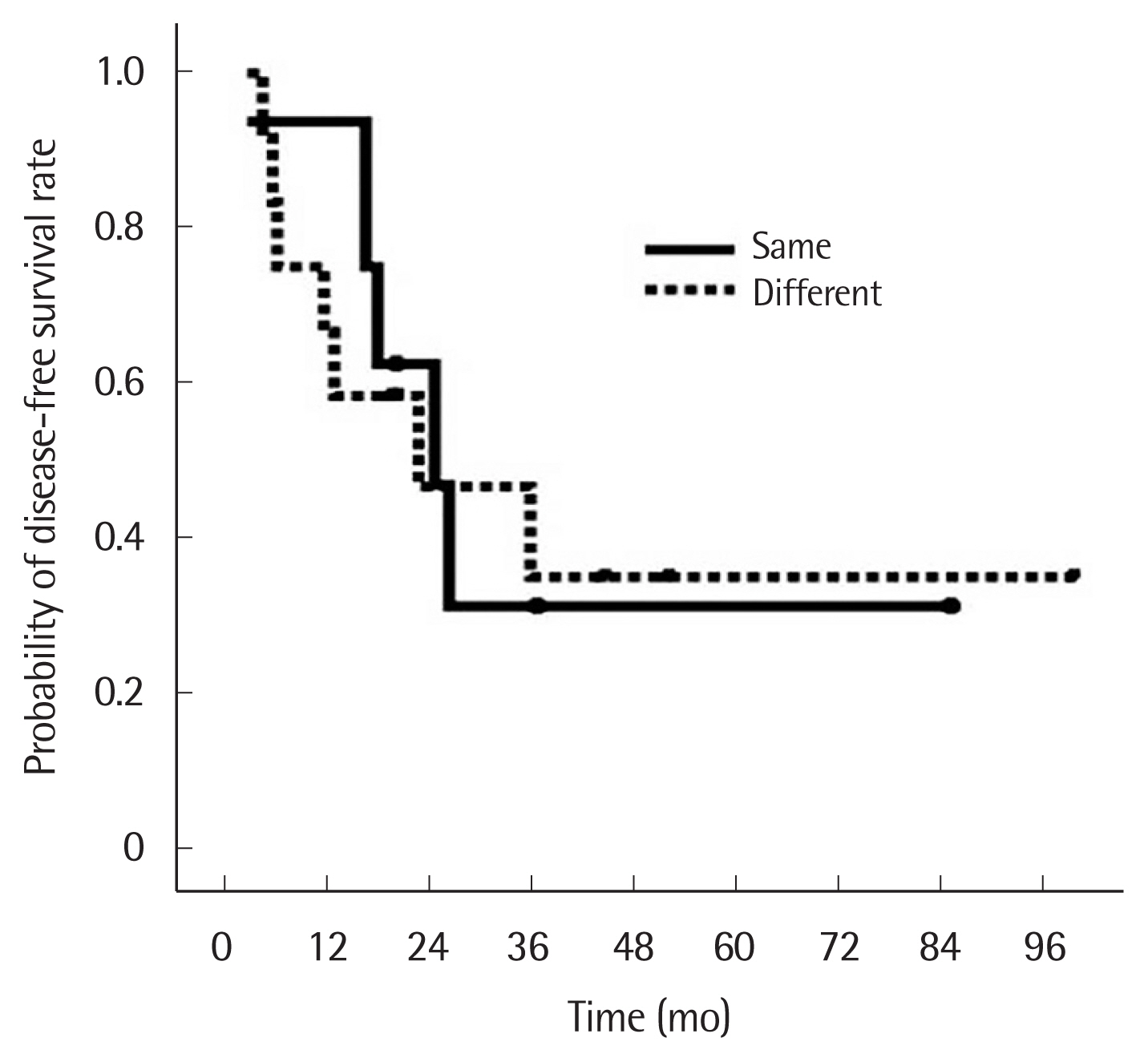
There were eight patients with the same histology for both tumors and 12 patients with different histology. Of the eight patients with the same histology, five had squamous cell carcinoma (SQCC) and three had adenocarcinoma: in all eight, IHC staining results showed patterns of p53 and EGFR expression that differed for each tumor. In the patients with different histology for each tumor, eight patients had SQCC+adenocarcinoma, two patients had large cell tumor+adenocarcinoma. Sixteen patients (80%) were in stage N0, one patient (5%) in stage N1, and three patients (15%) were in stage N2. The three patients in stage N2 had initial negative results on PET/CT scan and EBUS lymph node biopsy. Two of these patients had tumor cells at level 7 and one at level 5 (Table 2). There was no hospital mortality or major complications. Eight patients (40.0%) received adjuvant therapy. At the final follow-up, seven patients (35.0%) had died and 13 patients (65.0%) were alive. The median follow-up was 33.5 months (range, 6.1–102.3 months) for all patients and 41.6 months (range, 28.9–102.3 months) for the surviving patients. Recurrences occurred in 12 patients (60.0%), local recurrence in seven patients (35.0%), and distant metastasis in five patients (25.0%). The 1- and 5-year overall survival rate and disease-free survival rate were 80.0% and 63.3%, and 70.0% and 33.3%, respectively. The 5-year overall survival of patients with the same and different histology in each tumor was 56.3% and 66.7%, respectively, which was not significantly different (Fig. 1). The 5-year disease-free survival of patients with the same and different histology was also similar (31.3% vs. 35.0%) (Fig. 2).
On univariate analysis by log-rank test, there were no significant factors which included gender, age (>65 years old), smoking, tumor size, mSUV of tumors, location, cell type, and pathologic stage. The sublobar resection, including both sublobar resection+ sublobar resection and pulmonary resection more than lobectomy+ sublobar resection, did not have a negative effect on disease-free survival (Table 3).
DISCUSSION
Synchronous multiple cancerous lesions that have developed in primary lung cancer patients arise in two ways. One is IPM and the other is SPLC. Because IPM results from the hematogenous or lymphatic spread, surgical resection only is not the optimal treatment for IPM. However, in SPLC, at least two tumors have separate clonal origins; therefore, they can be treated by surgical resection alone. Synchronous multiple lesions are usually extensive and multifocal, occurring throughout the entire respiratory tree, and this phenomenon is due to similar exposure to carcinogens referred to as “field cancerization” [6]. The lung is also the most common site of hematogenous spread from metastasis of lung cancer. Therefore, it has been most important to differentiate double primary lung cancer from IPM in order to develop a treatment plan and predict the exact prognosis. The 8th TNM stage classification defined IPM in the same lobe as T3, in ipsilateral different lobes as T4, and in contralateral lobes as M1a. However, this staging system does not comment on double primary lung cancer. If multiple lesions showed different histologic types, they were designated as SPLC regardless of tumor location. In contrast, if multiple lesions showed the same histologic type, various conditions were considered. According to the criteria of Martini and Melamed [3], SPLC met the criteria as follows; lack of common lymphatic carcinoma, located in different lobes, and origin from carcinoma in situ. Although these definitions are far from perfect and their reliability has been questioned, they still provide the most useful and frequently referenced criteria for the definition of SPLC. However, many studies that have adhered to these criteria have shown a widely variable range of survival from 34% to 60.9% [2,5,7–9]. Taking into consideration that the prognosis of SPLC is much better than that of IPM, the studies that showed a high survival rate might have included more patients with genuine SPLC and vice versa. Therefore, many clinicians have attempted to designate a genuine SPLC group in order to determine the prognosis and prognostic factors more accurately. Beyond this, in some cases, SPLCs may have the same histologic type; then it was thought to be wrong to exclude the same histologic type in the evaluation of SPLC. Many methods have been used to show different origins in the same histologic type of SPLC. Among them, IHC staining has been found to be clinically relevant and easily performed in the clinical setting. IHC staining was a very popular method for revealing the origin of cancer, for example; when pulmonary malignant nodules in patients with extrathoracic malignancy have been detected, IHC staining may elucidate the origin; primary lung cancer or pulmonary metastasis. Based on these findings, two pulmonary tumors with different IHC staining patterns might indicate that these tumors are from different clones or origins and are, therefore, double primary lung cancer. Ono et al. [10] attempted to distinguish between two disease entities by analyzing the differential protein expression profiles and comparing the sum value of the difference in the expression ratio of four proteins (p53, p16, p27, and c-erbB2). They concluded that the profile of protein expression in cancer-related genes was considered to be a useful tool for distinguishing double primary lung cancers from IPM. Chang et al. [11] investigated the somatic mutations and altered expression of p53 and EGFR for clonality assessment and showed the occurrence of lymph node metastasis was more commonly observed in tumors with the same clonality and was associated with a poor 5-year survival rate. They suggested that analysis of somatic alterations in p53 and EGFR can significantly improve the clonality assessment and impact management of SPLC. Staining using CK-19, PE-10, and Ki-67 was useful in distinguishing double primary lung cancer from IPM in cases with a combination of adenocarcinoma and BAC [7]. Therefore, we included the patients with the same histology in each tumor in this study only if nodal metastasis did not exist and the pattern of IHC staining in each tumor was different. We excluded five patients with the same histology in each tumor without nodal metastasis because of the same pattern of IHC staining in each tumor.
To date, multiple GGO lesions have generated more confusion, in terms of SPLC. These multiple GGOs revealed multifocal BAC. BAC is a term that is no longer in use but used during half periods of our study. We used the former term until December 2011. Since then, the histopathological classification of the specimens was sorted by the regulations set by the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Lung Adenocarcinoma [4]. Adenocarcinoma in situ, minimally invasive adenocarcinoma, and some types of invasive adenocarcinoma have replaced the former term [4]. Though the exact mechanism was not clearly revealed, multiple GGOs have not been considered to be SPLC or IPM. Whether adenocarcinoma and BAC should be considered the same histology or not is basically another problem in understanding SPLC. Jung et al. [12] also considered the patients who had adenocarcinoma or BAC to have similar histology, because BAC was thought to be a precancerous lesion that could progress to adenocarcinoma. Therefore, in this study, if the patients had adenocarcinoma and BAC, they were considered to have the same histology.
As for the extent of lung resection, there are no definite guidelines and no standardized surgical methods. If two tumors existed ipsilaterally, most surgeons are not willing to perform pneumonectomy because of its high morbidity and mortality; instead, they prefer to perform sublobar resections such as wedge resection or segmentectomy for both lesions. In some cases, depending on the tumor sizes or locations in the lobe, performing lobectomy for the larger or more deep-seated tumor and sublobar resection for the smaller or more peripheral located tumor is a good option. If the tumors exist contralaterally, the extent of resection is determined based on pulmonary function; therefore, pulmonary resection more than lobectomy was done for both tumors in patients with good pulmonary function, while for patients with marginal pulmonary function, sublobar resection was done for at least one. In this study, the most common type of surgical procedure was pulmonary resection more than lobectomy with wedge resection, and pulmonary resection more than lobectomy for both lesions was performed for 25% of the patients.
Some studies reported that 5-year overall survival was approximately 35% [5,13]. Trousse et al. [13] reported that 2-year overall survival was 61.6%. In these studies, investigators did not use any other methods to show different origins when the histology of each tumor was the same. Compared with our study, the 5-year overall survival rate of our study was much better than the above studies mainly because of highly selected criteria for the same histology. We also tried to find the prognostic factor in SPLC patients. However, there was no prognostic factor for overall survival and disease-free survival.
Finley et al. [8] revealed that smaller tumor size, fewer pack-years of smoking, female, and overall pathologic stage were associated with improved survival by univariate analysis, and only female gender was an independent predictor of survival. Chang et al. [5] reported that the occurrence of lymph node metastasis was only a statistically significant prognostic factor. In this study, pathologic N status was not a significant prognostic factor. A possible explanation is the small number of cases. Future studies with a larger patient population may allow more precise analysis. In terms of surgical extent as a prognostic factor, Trousse et al. [13] noted that nonoptimal surgical treatment and performance of a pneumonectomy were independent predictors of poor long-term survival. However, given that the cell type of tumors or locations of tumors were not significant factors in any of these studies, might indicate that the patients were chosen very selectively.
Our study had some limitations. First, the study had a very small number of cases. However, we composed our study population highly selectively from among patients with multiple tumors by using strict selection criteria in order to reveal an accurate prognosis of SPLC. Second, due to the inherent nature of retrospective studies, there were no standardized strategies for the surgical methods used.
In conclusion, in strictly selected SPLC patients, the overall survival rate was not inferior to that of other literature studies. There was no significant prognostic factor for overall survival and disease-free survival. A large number of patients is needed for an appropriate and standardized selection process.