INTRODUCTION
Fitz-Hugh-Curtis syndrome (FHCS) is a perihepatitis due to intraperitoneal extension of pelvic inflammatory disease (PID) [1,2]. If sexually active women presenting with right upper quadrant (RUQ) abdominal pain visit a hospital, FHCS could be considered [2–5]. Although the accurate incidence of FHCS is currently unknown, the incidence of PID has been estimated to be 4% to 14% while that of PID in adolescent females can be as high as 27% [1,6,7]. Classical standard diagnosis of FHCS needs invasive procedures such as laparoscopy or laparotomy with detection of fibrous adhesion or identification of Neisseria gonorrhoeae or Chlamydia trachomatis in a specimen acquired from a capsular lesion of the liver [8–11]. However, for most cases, this syndrome can be well controlled by antibiotics [1–4,12]. Therefore, it is mostly diagnosed by non-invasive methods in recent years [13]. The diagnosis of FHCS is made by combining typical clinical symptom such as RUQ pain and typical imaging findings [14]. Although sonography can show widening of the right anterior renal space and loculation of fluid in the hepatorenal space, computed tomography (CT) is the most commonly used imaging tool for diagnosis of FHCS nowadays [15,16]. If arterial-phase CT reveals characteristic contrast enhancement of liver capsule, a confirmative diagnosis of FHCS can be made. Capsular enhancement seen on early arterial-phase images reflects increased blood flow or inflammation at the inflamed hepatic capsule [17]. In contrast, violin string-like adhesions are characteristics of the chronic phase of this disease while enhancement on delayed phase CT scan reflects early capsular fibrosis [18].
Although the use of arterial phase of CT has increased the diagnostic yield of FHCS and the value of dynamic CT in diagnosis of FHCS has been well researched, there are few reports regarding the relationship between clinical manifestation and capsular enhancement pattern. Therefore, the objective of the present study was to identify the clinical significance of capsular enhancement pattern of multidetector CT (MDCT) in patients with FHCS.
MATERIALS AND METHODS
1. Study populations
The present study protocol was reviewed and approved by the Institutional Review Board of Soonchunhyang University Bucheon Hospital (IRB approval no., 2016-07-007-001). This study retrospectively enrolled 86 females diagnosed with FHCS who had undergone MDCT between January 2005 and July 2017 at Soonchunhyang University Bucheon Hospital. Initially, a total of 121 female patients who visited the outpatient clinic or emergency room with suspected FHCS were retrieved. Among them, 35 patients were excluded for the following reasons: (1) monophasic contrast enhanced CT was performed (n=10), (2) no evidence of PID or gynecological symptoms (n=7), and (3) medical records of patients were insufficient (n=18). Finally, a total of 86 patients who were diagnosed as HFCS with MDCT examination were included in this study.
The diagnostic criteria of FHCS were as follows: the presence of RUQ pain, clinically diagnosed PID, hybrid capture test for Chlamydia trachomatis, and leukocytosis or tests for elevation of C-reactive protein (CRP) response to antibiotics.
2. Imaging study
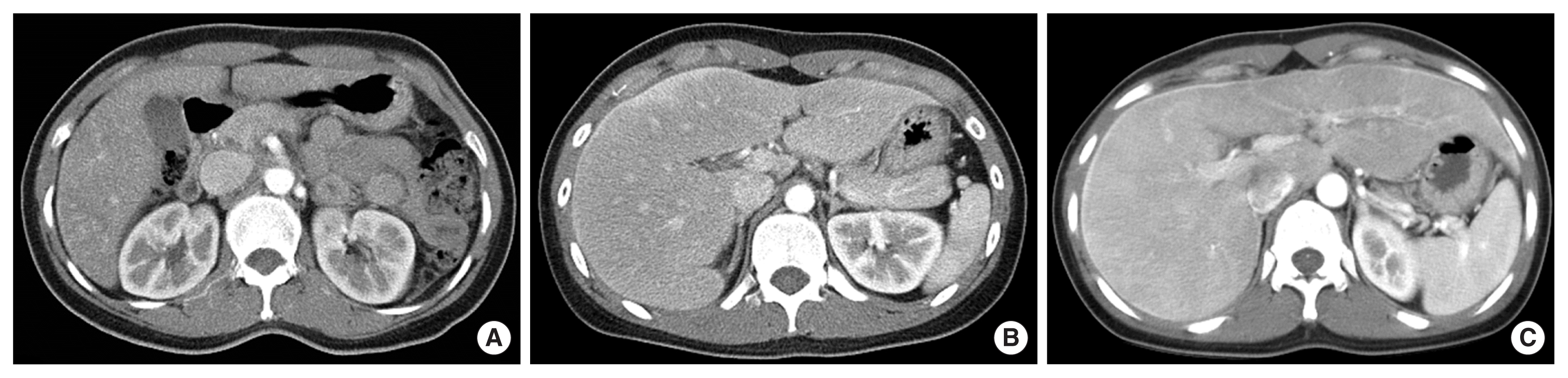
The hepatic capsular enhancement of dynamic CT findings was classified into three patterns: (1) pattern A, partial weak enhancement (Fig. 1A); (2) pattern B, partial strong or diffuse weak enhancement (Fig. 1B); and (3) pattern C, diffuse strong enhancement (Fig. 1C). The extent of enhancement was defined as partial (less than half of entire capsule) or diffuse (the above) while the intensity of enhancement was defined as weak (less intense than enhancement of portal vein) or strong (the above). It was reported by an independent radiologist who was blinded to clinical information. All CT images were analyzed by a single radiologist with more than 10 years of experience.
CT was performed with a 64-row MDCT scanner (LightSpeed VCT; GE Medical Systems, Milwaukee, WI, USA) or a 16-detector row CT scanner (Sensation 16; Siemens Medical Solutions, Erlangen, Germany), covering the top down to the floor of the liver. Patients were given intravenously 150 mL iomeprol (350 mg of iodine [/mL], Iomeron 350; Bracco, Milan, Italy) at a rate of 3 mL/sec using a high-pressure syringe. CT scanning began automatically when the threshold of abdominal aorta of the diaphragmatic dome level reached 100 Hounsfield unit, which was monitored by a software of contrast tracing. The scanning included three phases: arterial phase (15–25 seconds), portal phase (90 seconds), and delayed phase (180–210 seconds).
3. Statistics
Frequencies and percentages were used for descriptive statistics. Statistical differences between groups were investigated using χ2 test and Student t-test. Spearman’s analysis was used to investigate correlations between variables. Analysis of variance was performed to compare the duration of pain or length of stay between groups. To identify related factors associated with duration of pain or length of stay, linear regression analysis was used. Multivariate models were created using variables that were significant in a univariate analysis (P<0.05) and clinically relevant. All statistical analyses were performed using R ver. 3.3.3 (The R Foundation for Statistical Computing, Vienna, Austria) and IBM SPSS software ver. 21.0 (IBM Corp., Armonk, NY, USA). Statistical significance was defined at P<0.05.
RESULTS
1. Baseline characteristics
Baseline demographic and clinical characteristics of patients are listed in Table 1. All patients were women of childbearing age. Their mean age was 31.9±8.8 years (range, 19–51 years). The mean duration of pain in patients was about 4.2 days and the total length of stay was about 7 days. All patients recovered within 3 to 14 days. Approximately 85% of patients were prescribed antibiotics. Ceftriaxone was most common antibiotics, followed by ciprofloxacin. Pericapsular ascites known to indicate severity of the disease was observed in 26 patients (30.2%).
Although all patients were diagnosed with PID on clinical and imaging studies, positive rates of chlamydia and gonococcus were low. Chlamydia was the most common causative organism. It was found in 10 patients (11.6%). Patients had a slight increase in white blood cell count (WBC) and an increase in neutrophil fraction. CRP level was at 7.1 mg/dL on average. It was increased in all patients. However, liver function tests and serum creatinine levels were within normal ranges.
2. Classification according to the degree of enhancement
Patients were classified into three groups according to the degree of enhancement of CT findings: weak, moderate, and strong groups (Table 1). As mentioned above, the weak group had a partial weak enhancement (Fig. 1A, pattern A), while the moderate group had a partial strong or diffuse weak enhancement (Fig. 1B, pattern B). Patients in the strong group had a diffuse strong enhancement (Fig. 1C, pattern C). The duration of pain was significantly increased in the order of weak, moderate, and strong groups (2.9 vs. 4.3 vs. 5.7 days, P<0.001 for trend). Duration of hospital stay was also increased in the same pattern (6.1 vs. 7.2 vs. 7.7 days, P=0.017 for trend) (Fig. 2). The rate of pericapsular ascites was also significantly increased in the order of weak, moderate, and strong groups (3.6% vs. 28.6% vs. 65.2%, P<0.001). However, age, selection of antibiotics, causative organisms, WBC, or CRP was not associated with the degree of enhancement.
3. Related factors determining length of hospital stay
Next, we examined which factors affected the total length of hospital stay in patients with FHCS through linear regression analyses (Table 2). Univariate analysis showed that the degree of enhancement and pain duration was related to total length of hospital stay. In multivariate analysis, strong enhancement pattern group significantly increased the length of hospital stay (beta coefficient [BE], 2.19; 95% confidence interval [CI], 0.63–3.76, P=0.007), even after adjusting for duration of pain.
4. Related factors determining duration of pain
Finally, we examined which factors affected the duration of pain in patients with FHCS by linear regression analyses (Table 3). Univariate analysis results showed that degree of enhancement, presence of pericapsular ascites, use of ceftriaxone, and pain duration were related to duration of pain. In multivariate analysis, degree of enhancement was related to pain duration dose-dependently (moderate enhancement: BE, 1.33; 95% CI, 0.45 to 1.60, P<0.001; strong enhancement: BE, 2.30; 95% CI, 1.79 to 2.81, P<0.001) after adjusting for other factors. Similar to the previous analysis, hospital stay was closely related to duration of pain in multivariate analysis (BE, 1.25; 95% CI, 0.98 to 1.53; P<0.001). In addition, the use of ceftriaxone significantly reduced pain duration (BE, 0.96; 95% CI, 0.36 to 1.55; P=0.002).
DISCUSSION
The prevalence of FHCS is increasing with recent liberalization of sex life compared to the past. Most cases of FHCS can be treated with antibiotics. If not properly treated early, significant morbidity and long-term complication can occur [19,20]. Therefore, it is important to determine the severity of FHCS during initial diagnosis. Our results showed that the degree of CT enhancement was closely related to its clinical severity index including hospital admission duration and duration of pain.
Many studies have shown that characteristic imaging finding of FHCS is an ehancement of the liver capsule in arterial phase of CT scan [2,4,13]. Previously, many invasive methods such as laparoscopy have been used to diagnose FHCS. However, most of these methods are now replaced by non-invasive methods such as CT [15,17,18]. In fact, CT finding and pathological FHCS staging are highly correlated [13]. In addition, CT can help distinguish PID from other diseases that can cause enhancement of liver capsule. However, there is little research on the severity and grading of FHCS. Wang et al. [21] have used liver capsule thickening shape and range on CT and magnetic resonance imaging examinations for grading FHCS. Kim et al. [18] have proposed a classification system using ehancement site, shape, and thickness changes of liver capsule enhancement using CT scan. However, these classifications are complicated. In addition, they have not been widely applied in clinical practice. The classification method used in this study is advantageous in that it can be applied relatively simply by using ehnacement degree and range only.
We first hypothesized that the degree of ehancement would be directly related to the degree of inflammation. However, the extent of enhancement in CT showed no significant correlation with WBC or CRP level, a biochemical marker of inflammation. This result is consistent with other studies [1]. In previous studies, ESR was elevated in only a few patients while it was within normal range in most patients [1,22]. First, FHCS has relatively limited inflammation of peritoneal and perihepatitis. It is possible that the level of systemic inflammation has not increased significantly in these locualted inflammation. For example, in the case of tuboovarian abscess which is the most severe form of PID, the perihepatitis may not be as severe as the sealing effect and may not have a direct correlation with systemic inflammation biomarker [23].
However, CT enhancement grading was closely related to the duration of pain and the entire hospital stay known to be important clinical indicators. In other words, it is impossible to determine the severity of FHCS disease by laboratory finding alone. Image findings are essential for severity determination. Therefore, in patients with strong enhancement of initial CT, it is necessary to administer antibiotics as soon as possible and actively control inflammation and pain by using NSAIDs. This active treatment can result in reduction in overall hospitalization and medical cost. In fact, it has been reported that the faster the appropriate antibiotic is administered, the faster the symptom control and the lower chance of complication [24].
Neisseria and Chlamydia are the two most common etiological organism causing FHCS. However, the difference in enhancement pattern was not significant accroding to etiological organism in this study. The causative organism of FHCS is mainly dependent on past research. In the past, the ratio of Neisseria and Chlamydia reached 90% [25,26]. However, it seems that FHCS is caused more by organisms other than Neisseria and Chlamydia recently due to liberalization of sexual life and diversification of STD [25,27,28].
In our study, pain duration was significantly shorter when ceftriaxone was used. In the case of STD, coexisting cases are frequent. If suspected bacteria are not clearly identified, it is a principle to treat the possible bacteria simultaneously [29]. The results of this study suggest that ceftriaxone has a wider coverage than other antibiotics (tetracycline, macrolide, etc.). Thus, it may improve PID symptoms more effectively.
In conclusion, the CT enhancement pattern in FHCS is closely related to its clinical severity and the course of this disease. Patients with strong enhancement pattern may require faster treatment and active pain control. Additional research is needed to determine the relationship between the enhancement pattern and pathogens or choice of appropriate antibiotics in the future.