INTRODUCTION
Amyloidosis is a disorder characterized by abnormal folding of proteins; the deposition of these proteins which compose insoluble fibrils disrupts tissue structure and function. Amyloidosis can be occurred as primary or secondary, and the deposition of amyloid fibrils can be localized or systemic [1]. Amyloidosis can be occurred secondary to chronic inflammation or chronic infection such as inflammatory bowel disease [2], rheumatoid arthritis, tuberculosis, myeloma, or bronchiectasis [3]. Amyloidosis is not common; however, it can cause serious complication in inflammatory diseases, but it is a serious complication [4,5]. It is known from clinical experience that amyloidosis only rarely appears as amyloid goiter accompanied by enlargement of the thyroid. After the first reported case of renal amyloidosis accompanying Crohn’s disease was reported in 2006 [6], a case of gastrointestinal amyloidosis accompanying Crohn’s disease was reported in 2011. In Korea, however, any case of systemic amyloidosis in Crohn’s disease diagnosed based on amyloid goiter has not been reported to-date [7]. We report here a case of secondary amyloidosis in which amyloid goiter and gastrointestinal amyloidosis occurred simultaneously in a patient with Crohn’s disease.
CASE REPORT
A 74-year-old female patient was admitted to Soonchunhyang University Hospital because of general weakness and poor oral intake. She had been diagnosed with Crohn’s disease 3 years earlier, and was being treated with 5 mg prednisolone and 3,000 mg mesalazine at the time of admission. Her abdominal pain worsened during the last 30 days. 14 days before hospitalization, we conducted abdominal computed tomography (CT), an enteroenteric fistula was observed in addition to deterioration of her Crohn’s disease. 10 days before hospitalization, we began to administer 287 mg of infliximab to the patient. Because she had been diagnosed with hypertension 6 years earlier and coronary artery disease 4 years earlier, she was taking 100 mg of aspirin, 120 mg of isosorbide dinitrate, and 90 mg of diltiazem. When hospitalized, she had a blood pressure of 120/80 mmHg, pulse of 88 beats per minute, and body temperature of 36°C. She was alert but appeared chronically ill. Unlike the follow-up observations during the previous outpatient treatment, anterior-neck diffuse goiter was found, but there was no tenderness. In the abdominal examination, it was found that the bowel sound was decreased, and there was tenderness around the navel. Laboratory examination demonstrated leukocytes 12,000/mm3, hemoglobin 9.2 g/dL, platelets 142,000/μL, aspartate aminotransferase 23 U/L, alanine aminotransferase 12 U/L, Na 138 mmol/L, K 4.5 mmol/L, Cl 100 mmol/L, creatinine 1.16 mg/dL, erythrocyte sedimentation rate 48 mm/hr, and high-sensitivity C-reactive protein 22.80 mg/dL. A thyroid function test showed that T3 was 12.55 ng/dL (normal range, 60 to 190 ng/dL), free T4 1.2 ng/dL (normal range, 0.7 to 2.0 ng/dL), thyroid stimulating hormone 12.88 μIU/mL (normal range, 0.25 to 0.4 μIU/mL), thyroid stimulating immunoglobulin 0.90% (normal range, 0% to 15%), anti-thyroid peroxidase antibody 0.14 U/mL (normal range, 0 to 0.3 U/mL), and anti- thyroglobulin antibody 0.44 U/mL (normal range, 0 to 0.3 U/mL). Based on these findings, we diagnosed the patient with hypothyroidism.
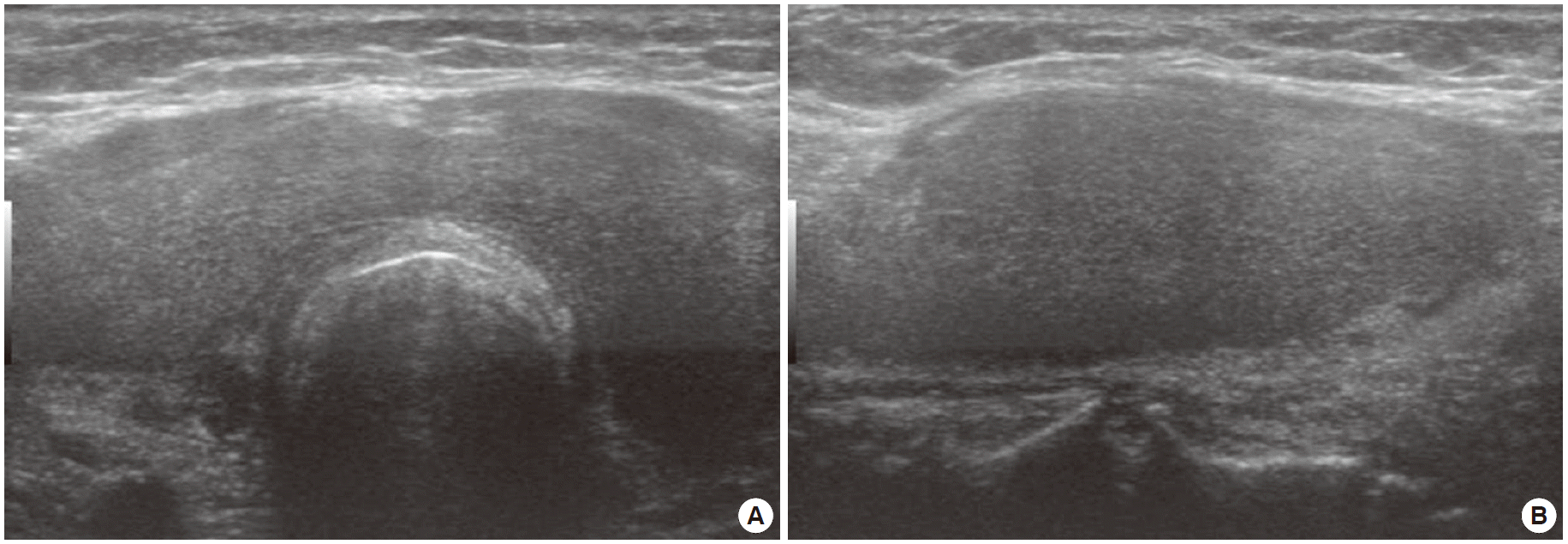
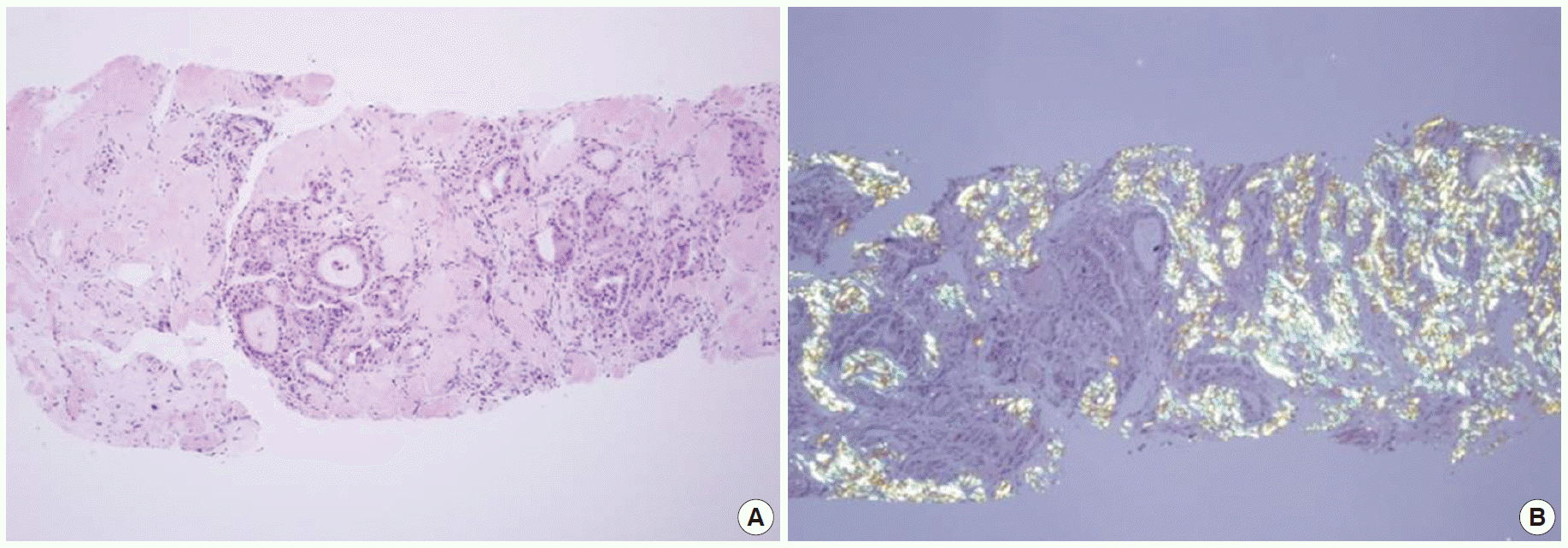
By thyroid ultrasonography, diffuse hypertrophy was found in both thyroid glands (Fig. 1), which required that thyroiditis be distinguished from lymphoma. In an additional neck CT examination, hypertrophy of the thyroid glands was observed, with mixed contrast enhancement (Fig. 2), so an ultrasonography-guided needle biopsy was carried out to discriminate lymphoma. Pathologic examination showed fibrous stroma and entrapped medium-sized follicles lined by tall cuboidal cells, some of which were filled with thin colloid. There was deposition of amorphous eosinophilic material, which was birefringent on polarizing microscopy after congo red staining. The patient was diagnosed with amyloid goiter (Fig. 3).
Because of diagnosis of amyloid goiter and worsening of poor oral intake, upper gastrointestinal (UGI) endoscopy was performed to confirm the amyloid in the gastrointestinal tract. Edematous and hyperemic mucosal changes were observed in the entire stomach, and small ulcers with a slightly whitish mucosal change were observed in the duodenum. Random biopsies in both of the gastric body and the duodenum were performed (Fig. 4). In the pathologic examination, amyloid deposition was observed after congo red staining (Fig. 4). We carried out a pathologic review of a sigmoidoscopic biopsy conducted before infliximab was administered and confirmed amyloid deposition after congo red staining. In an immunoelectrophoretic examination of blood and urine conducted to discriminate primary amyloidosis, there were no specific findings. But since polyclonal gammopathy was observed by immunofixation, the patient was diagnosed with secondary amyloidosis involving both the thyroid and the gastrointestinal tract accompanying Crohn’s disease. It was planned that infliximab would be constantly administered to the patient for the treatment of systemic secondary amyloidosis. But she had a blood pressure of 90/60 mmHg, body temperature of 38.2°C, rapidly decreased renal function, and identified methicillin resistant staphylococcus epidermis from blood culture on 32th day of hospitalization. We treated septic shock with hydration, vancomycin and meropenem antibiotics. But due to sepsis, she expired on the 35th day of hospitalization while receiving treatment.
DISCUSSION
Amyloidosis occurs when an insoluble fibrous protein, amyloid, is deposited beside cells, and may induce failure of the involved organs. As a disease related to chronic inflammation or an infectious disease, secondary amyloidosis most commonly involves the kidneys, and it also may occur in the gastrointestinal tract, liver, autonomic nerve tissue, and even the heart, albeit rarely [8]. Amyloid infiltration in the thyroid was first reported by von Rokitansky in 1855 [9]. Although amyloid was found in the thyroid of 50% to 80% of primary and secondary amyloidosis patients [10], it rarely appears clinically as diffuse goiter, which von Eisenberg named ‘amyloid goiter.’ One characteristic of amyloid goiter is its rapid growth, which makes to need to discriminate from a malignant tumor [8]. Patients with amyloid goiter rarely have thyroid dysfunction, but some studies have reported cases accompanied by hyperthyroidism or hypothyroidism [11]. In this case, as the patient had thyroid goiter that occurred suddenly, and had a suspicious of a malignant tumor, we performed thyroid ultrasonography and neck CT and observed diffuse thyroid goiter on both sides. Afterward, by ultrasonography-guided needle biopsy conducted for confirmation, amyloid deposition was observed in the thyroid. Accordingly, when the thyroid is rapidly enlarged in patients with chronic inflammatory diseases, such as inflammatory bowel disease, rheumatoid arthritis, and sarcoidosis, it is necessary to determine the presence of amyloid goiter.
In this case, the patient was found to have abdominal pain and decreased ingestion, and when she was confirmed to have amyloid goiter, UGI endoscopy was carried out to find out whether amyloid involved the gastrointestinal tract. In random biopsies of both the gastric body and the duodenum, amyloid deposition was observed after congo red staining. Based on these results, the patient was diagnosed with gastrointestinal amyloidosis.
In a large-scale study of secondary amyloidosis occurring in inflammatory bowel disease, its prevalence was 0.9% in Crohn’s disease and 0.07% in ulcerative colitis. In gastrointestinal tract-involved amyloidosis, symptoms may include macroglossia, vesicular lesions, ulcers, and xerostomia in the oral cavity; motor disturbance, hematemesis, achalasia, aperistalsis, portal hypertension, and esophageal varices in the esophagus; and early satiety, nausea, vomiting, abdominal pain, and hematemesis if the stomach is involved [1,12]. The small intestine is the most common area of amyloidosis, and due to intestinal hypoperistalsis and intestinal amyloid deposition, symptoms such as diarrhea, intestinal obstruction, intestinal perforation, and intestinal bleeding can also occur [1,13]. When the large intestine is involved, amyloidosis may also cause intestinal obstruction, intestinal perforation, and intestinal bleeding [1]. As mentioned earlier, gastrointestinal amyloidosis has such varied symptoms and nonspecific findings that it is difficult to diagnose; indeed, radiologic test results and endoscopic findings are also non-specific [14]. Using a polarization microscope, congo red staining after biopsy of organs suspected of being involved will reveal an apple green color, which indicates deposition of amyloid fibroid material.
One of the principles of treatment of secondary amyloidosis is to reduce the creation of serum amyloid A protein [15]. No effective treatment has yet been developed, but it has been reported that inflammation-suppressive or immunosuppressive therapies, mainly using tumor necrosis factors such as cytokines, are efficacious [16]. In this case, the patient with Crohn’s disease had deteriorated, and an enteroenteric fistula was discovered, so infliximab was administered. After this, we were diagnosed with amyloidosis. After the patient had been diagnosed with amyloidosis, it was planned that infliximab would be administered again, but because of the general deterioration due to sepsis, this did not occur.
Amyloidosis accompanying Crohn’s disease is rare, and its symptoms and endoscopic findings are non-specific, which may delay diagnosis. Amyloidosis can involve several organs such as the kidney, gastrointestinal tract, heart, liver, and thyroid, and when a patient receives treatment lately the prognosis may be poor. Therefore, it is necessary to confirm symptoms attentively during follow up observations, and when abnormal findings are found in a physical examination such as cervical goiter, specific tests should be carried out to determine whether systemic amyloidosis is present.