SMS 2011 December;17(2):58-64.
Published online 2011 December 30 |
| Copyright ⓒ 2010 Soonchunhyang Medical Science
|
| The Effect of Metabolic Syndrome on Myocardial Contractile Reserve during Exercise in Non-Diabetic Hypertensive Subjects |
| Se-Hun Kim1, Hye-Sun Seo2, Nae-Hee Lee2, Jaehuk Choi1, Tae Hoon Ha2, Jon Suh2, Youn-Haeng Cho2
|
1Department of Internal Medicine, Soonchunhyang University Bucheon Hospital,
2Division of Cardiology, Department of Internal Medicine, Soonchunhyang University Bucheon Hospital, Bucheon, Korea |
| Corresponding Author: Hye-Sun Seo , Tel: +82-32-621-5131 , Fax: +82-32-621-5016 , Email: haesun@schmc.ac.kr
|

|
ABSTRACT
|

|
|
|
Objective: Metabolic syndrome (MS) is associated with increased left ventricular (LV) mass and diastolic dysfunction. This study uses relatively load-independent Doppler tissue echocardiography to examine whether MS is associated with decreased longitudinal contractile reserve during dynamic exercise. Methods: A total of 112 patients with relatively well-controlled, treated hypertension who complained of exertional dyspnea were enrolled (average age, 56.7±10.5 years). Fifty-six were non-diabetic patients with MS (group 1), and 56 were age-sex matched hypertensive patients without MS (group 2). Exercise stress echo was performed using a symptom-limited, multistage, supine bicycle exercise test. Multiple Doppler parameters were obtained at baseline, at each stage of exercise. Results: There was no significant difference between the two groups in terms of age, gender, and hemodynamic variables. E/E’, an index of LV filling pressure, was significantly higher in the MS group at rest and during exercise. The longitudinal contractile reserve, the change in S’ (longitudinal tissue velocity) from baseline to peak exercise, was significantly lower in the MS group (2.00±1.65 vs. 2.90±1.66, P=0.015). Multiple regression analysis showed independent association of MS with longitudinal contractile reserve when controlled for confounding factors, such as LV mass index, gender, blood pressure, and age (β=-0.235, P=0.035). Conclusion: Longitudinal contractile reserve was reduced in MS patients compared to others, although both groups demonstrated similar longitudinal contractile function at rest. We present the first demonstration that metabolic syndrome is independently associated with LV systolic dysfunction during exercise in hypertensive patients. |
|
Keywords: Metabolic syndrome; Longitudinal function; Contractile reserve |
|

|
INTRODUCTION
|

|
|
Metabolic syndrome (MS) is characterized by a group of cardiovascular risk factors, including obesity, hypertension, dyslipidemia, and disturbed glucose metabolism that are associated with an excess risk of cardiovascular disease
[1-3]
. Previous studies have demonstrated a significant correlation between obesity and insulin resistance and adverse left ventricular (LV) structure and function and an increased risk of congestive heart failure
[4-8]
. These findings suggest that increased cardiovascular risk may be mediated by LV dysfunction. Several studies have shown that subclinical diastolic dysfunction is evident in most patients with obesity and MS
[9,10]
, also with either increased or no interval change in LV systolic function at rest [9,11,12]. A study by Sasso et al.
[9]
demonstrated a reduced increase in ejection fraction in obese subjects during dynamic exercise using radionuclide ventriculography; however, ejection fraction is a load-dependent measurement of systolic function that may not accurately reflect the subtle changes of early myocardial dysfunction. Moreover, no studies have been performed on the effect of MS on myocardial contractile reserve during dynamic exercise.
Therefore, we tried to determine whether MS is associated with decreased longitudinal contractile reserve with exaggeration of diastolic dysfunction during dynamic supine bicycle exercise, using relatively load-independent Doppler tissue echocardiography. |

|
MATERIALS AND METHODS
|

|
|
1. Subjects
A total of 212 patients (aged 36 to 76 years; mean age, 56.7±10.5; male, 86/40.6%) with relatively well-controlled, treated hypertension and who complained of exertional dyspnea, were initially enrolled in this study after providing informed consent. Fifty-six patients satisfied the Asian modified criteria of MS
[13,14]
. From the remaining 156 patients, 56 non-diabetic patients, matched for age and sex, were selected as controls. Patients with diabetes were excluded due to the possible confounding effect of diabetes on myocardial function. Patients with any of the following were also excluded: valvular heart disease, peripheral vascular disease, significant systemic disease, history of inflammatory disease, symptomatic cerebrovascular disease (including previous transient ischemic attack within the previous six months), history of significant coronary artery disease, a clinically significant atrioventricular conduction disturbance, history of atrial fibrillation or other serious arrhythmia, history of congestive heart failure, liver cirrhosis, severe hypertension (>180/110 mmHg), serum creatinine >1.4 mg/dL, pregnancy, or lack of appropriate contraception use in women of childbearing potential. Use of the following drugs was not permitted during the study: antihypertensive medications other than the study agents, lithium, major psychotropic agents, oral steroids, daily nonsteroidal anti-inflammatory drugs, high-dose acetylsalicylic acid, or drugs with possible interactions with the study drugs (digoxin, cimetidine, rifampin, erythromycin, itraconazole, grape-fruit juice). All patients underwent a complete physical examination and laboratory assessment at the time of enrollment.
2. National Cholesterol Education Program (NCEP) Criteria of Metabolic Syndrome (MetS) and Modified NCEP Criteria for Asians
The NCEP adult treatment panel III (ATP III) guidelines classify individuals as having MS if they possess three or more of the following criteria:
High blood pressure (BP): systolic BP>130 mmHg and/or diastolic BP>85 mmHg or use of BP-lowering drugs.
Hyperglycemia: fasting plasma glucose≥6.1 mmol/L (110 mg/dL) or use of glucose-lowering drugs.
Hypertriglyceridemia: fasting plasma triglycerides≥1.69 mmol/L (150 mg/dL).
Low high-density lipoprotein-cholesterol (HDL-C): fasting HDL-C<1.04 or 1.29 mmol/L (40 or 50 mg/dL) for males and females, respectively.
Central obesity: The World Health Organization Western Pacific Region has recognized the disproportionate contribution of obesity to the development of cardiovascular risk factors in Asians and has provisionally lowered the classification of central obesity to waist circumference >80 cm in females or >90 cm in males and/or body mass index (BMI) ≥25 kg/m2 for both sexes.
3. Two-Dimensoinal (2D) and Doppler Echocardiography
Echocardiography was performed using an ultrasound system (Vivid-7, GE Vingmed, Horten, Norway) with a 2.5-MHz transducer. Standard 2D measurements (end-diastolic and end-systolic dimensions, ventricular septum and posterior wall thickness, left atrial volume index, LV mass [LVM] index, LV outflow tract) were made with the patient in the left lateral position. The LV ejection fraction was calculated by the modified method of Quinones et al.
[15]
. Tissue Doppler imaging (TDI) was performed in an apical 4-chamber view with the sample volume placed at the septal border of the mitral annulus. To evaluate LV filling pressures, the ratio mitral inflow peak velocity (E)/early diastolic tissue velocity of the mitral annulus (E’) was calculated.
4. Exercise Stress Echocardiography
Exercise stress echo was performed using a symptom-limited, multistage, supine bicycle exercise test with a variable load bicycle ergometer (Medical Positioning Inc., Kansas City, MO, USA), as described previously
[16]
. Briefly, after obtaining images at rest, patients pedaled at constant speed, beginning at a workload of 25 W and adding an incremental workload of 25 W every 3 minutes. From the apical window, a 1- to 2-mm pulsed Doppler sample volume was placed at the mitral valve tip, and mitral flow velocities from 5 to 10 cardiac cycles were recorded. The mitral inflow velocities were traced, and the following variables were obtained: early mitral peak velocity (E), late velocity (A), and the deceleration time of the E wave velocity. Mitral annulus velocity was measured by Doppler tissue imaging using the pulsed wave Doppler mode. Annular systolic tissue velocities (S’) and E’ were measured from the apical 4-chamber view with a 2- to 5-mm sample volume placed at the septal corner of the mitral annulus. Measurements were recorded with simultaneous electrocardiography at sweep speeds of 50 to 100 mm/sec. Each measurement was made at baseline at each stage of exercise and during recovery. Due to the high incidence of fusion of E’ and A’ during exercise at workloads greater than 50 W, parameters of diastolic function were assessed at baseline, 25 W, and 50 W. Due to the fact that measurement of S’ was readily obtainable during peak exercise, exercise contractile reserve was assessed at peak exercise. Two-dimensional echocardiographic images were recorded at rest and at each stage of exercise and digitally stored for further analysis. Measurements were performed off-line by a single investigator who was unaware of the status of the study patients. Patients who demonstrated evidence of overt myocardial ischemia during exercise, such as significant ST segment change or development of regional wall motion abnormality, were excluded from analysis.
5. Statistical Analysis
Values were expressed as mean±SD. P-value<0.05 is considered significant. Comparison of the dichotomous variables was performed using the chi-square analysis. Comparison of continuous variables between the two study groups was performed using the Student’s t-test. Independent clinical predictors of exercise associated elevation of S’ (myocardial contractile reserve) were determined using multiple linear regression analysis. Statistical analysis was performed with the SPSS ver. 11.0 (SPSS Inc., Chicago, IL, USA). |

|
RESULTS
|

|
|
Among the 112 patients who were enrolled in this study, 56 were non-diabetic patients with MS (group 1), while 56 were age-sex-mat-ched hypertensive patients without metabolic syndrome (group 2).
There were no significant differences between the two groups in terms of age, gender, baseline BP, or total cholesterol level
(Table 1)
. However, patients in group 1 demonstrated significantly higher BMI, serum triglyceride, and fasting blood sugar level and significantly lower HDL cholesterol level compared to group 2
(Table 1)
. Although there was no significant difference in the LV dimension and baseline LV ejection fraction between groups 1 and 2, group 1 demonstrated significantly higher relative wall thickness and LVM index
(Table 1)
. There were no significant differences between the two groups with respect to the types of anti-hypertensive medications administered and mean systolic and diastolic blood pressure.
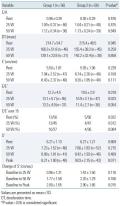
Hemodynamic variables, such as systolic BP, diastolic BP, heart rate, and ejection fraction during peak exercise were not significantly different between the two groups. Fifty patients in each group performed exercise of more than 50 W with no significant difference in exercise duration and metabolic equivalents achieved during exercise
(Table 2)
.
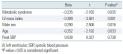
Comparison of the Doppler echocardiographic parameters at baseline and during exercise at 25 W and 50 W showed no significant difference in terms of the E/A ratio, deceleration time, or E’. However, E/E’, an index of LV filling pressure, was significantly higher in patients with MS at rest and at 25 W of exercise, with borderline statistical significance at 50 W. In addition, the proportion of patients with E/E’ greater than 15 was higher among patients with MS at rest and at 25 W and showed a tendency to be higher at 50 W. Although the absolute value of S’ was not significantly different between the two groups at baseline, 25 W and 50 W, there was a tendency for a lower S’ value in the MS group. The change of S’ from baseline, the longitudinal contractile reserve, also tended to be lower in the MS group at 25 W and 50 W of exercise. At peak exercise, the longitudinal contractile reserve was significantly lower in the MS group (2.00±1.65 vs. 2.90±1.66, P= 0.015) (
Table 3
, Fig. 1).
Multiple regression analysis was performed to determine the independent association of MS with longitudinal contractile reserve when controlled for confounding factors, such as LVM index, gender, BP, and age. The results show that LVM index (β=-0.389, P= 0.001), male gender (β=0.290, P=0.016), age (β=-0.252, P=0.033), and MS (β=-0.235, P=0.035) were independently associated with the degree of longitudinal contractile reserve
(Table 4)
. |

|
DISCUSSION
|

|
|
The novel finding of our study was that metabolic syndrome influences both systolic and diastolic function during exercise. Longitudinal contractile reserve was reduced in hypertensive patients with MS compared to patients without MS, although both groups demonstrated similar longitudinal contractile function at baseline. Moreover, E/E’, an index of diastolic function and LV filling pressure, was significantly increased at each stage of exercise in patients with MS. This demonstrates that increased filling pressure and diastolic dysfunction was maintained during dynamic exercise as well as in the resting state, as shown in previous studies [5,10,17].
1. MS and Cardiac Remodeling
Several components of the insulin resistance syndrome are associated with increased LV relative wall thickness, LV concentric remodeling
[18]
, and LV hypertrophy [19,20]. Components of MS were associated with increased LVM in a large cohort of blacks in the Atherosclerosis Risk in Communities Study
[21]
; however, up to 23% of the population in that study had frank diabetes, a well-known determinant of LV hypertrophy
[22]
. Therefore, the possibility of diabetes playing a significant role in the association between metabolic factors and LVM could not be excluded. The pre-sent study directly explored the association between MS and cardiac structure and function in non-diabetic hypertensive patients, thus excluding the influence of diabetes.
In this study, we demonstrated a close linkage between metabolic components and LV geometric changes. LV concentric remodeling was evidenced by increased LVM index, increased LV wall thickness, increased relative wall thickness, and normal LV chamber size. This finding is very important, since LV concentric remodeling is associated with increased cardiovascular morbidity and mortality in the absence of definite LV dysfunction
[23]
.
2. MS Syndrome and LV Dysfunction
Obesity and insulin resistance syndrome are known to increase the risk of congestive heart failure. Even in patients without overt cardiovascular disease, obesity and MS has been associated with subclinical myocardial dysfunction [5-8]. Although subclinical diastolic dysfunction is evident in most patients with obesity and MS
[9,10]
, the effect of metabolic syndrome on systolic function is not clear [9,11,12], with some studies reporting an increase in LV systolic function associated with subclinical diastolic dysfunction
[9]
. Although conventional measures of LV systolic function, such as left ventricular ejection fraction, may be preserved in patients with subclinical myocardial dysfunction and diastolic dysfunction, this may reflect radial compensation for a decrease in longitudinal systolic dysfunction [24-26]. Due to the fact that longitudinal fibers that anatomically connect the mitral annulus are mainly subendocardial fibers, they are more sensitive to early changes of ischemia and fibrosis [26,27]. Therefore, measurement of systolic and diastolic mitral annulus tissue velocity may be a sensitive me-thod for the detection of subclinical dysfunction of longitudinal myocardial contraction [28,29].
Obesity and insulin resistance are characterized by alterations in myocardial metabolism, further defined by derangement in fatty acid metabolism and metabolic deficiency
[5]
.
The increased myocardial oxygen consumption that is associated with insulin resistance syndrome may be exaggerated during dynamic exercise. In addition, obesity and MS are associated with increased LVM that may contribute to myocardial dysfunction. Although an increase in LVM contributes significantly to decreas-ed longitudinal contractile reserve, the presence of MS itself was independently associated with reduced contractile reserve even when controlled for confounding variables, such as age, LVM index, and gender. This demonstrates that the cluster of cardiovascular risk factors acts in concert to adversely affect myocardial function during exercise. Obesity and MS are associated with impaired coronary endothelial function and decreased coronary flow reserve may be associated with subendocardial ischemia during exercise [30,31]. Increased activation of the renin-angiotensin-aldosterone system in patients with MS may also have a significant role in adverse cardiac remodeling and dysfunction
[32]
.
This study was performed in treated hypertensive subjects, so the results may be confounded by the influence of BP medications. However, there were no significant differences in the proportion of different classes of medications that were administered
(Table 1)
, and BP medication was assumed to have a minimal effect on the results of this study. The lack of an effect on hemodynamic variables at exercise and exercise duration also minimizes their influence on the results of this study
(Table 2)
. Another limitation of this study is that the presence of subclinical atherosclerotic heart disease cannot be completely ruled out by non-invasive methods. We tried to minimize this confounding effect by excluding patients with regional wall motion abnormality during exercise echocardiography and/or patients with significant ST segment change during exercise. |
|

|
FIGURES
|

|
|

|
Fig.1
Annular systolic tissue velocity at rest and during exercise (longitudinal contractile reserve). |

|
|

|
TABLES
|

|
|

|
Table.1
Baseline clinical and echocardiographic characteristics |

|

|
Table.2
Comparison of hemodynamic variables at rest and during exercise |

|
|
Table.3
Comparison of Doppler echocardiographic variables at rest and during exercise |

|
|
Table.4
Independent determinants of longitudinal contractile reserve by multiple linear regression (R2=0.429) |

|
|
| |

|
REFERENCE
|

|
|
|
1.
|
Isomaa B, Almgren P, Tuomi T, Fors?n B, Lahti K, Niss?n M, et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 2001;24:683-9. |
|
2.
|
Lakka HM, Laaksonen DE, Lakka TA, Niskanen LK, Kumpusalo E, Tuomilehto J, et al. The metabolic syndrome and total and cardiovascular disease mortality in middle-aged men. JAMA 2002;288:2709-16. |
|
3.
|
Schillaci G, Pirro M, Vaudo G, Gemelli F, Marchesi S, Porcellati C, et al. Prognostic value of the metabolic syndrome in essential hypertension. J Am Coll Cardiol 2004;43:1817-22. |
|
4.
|
Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, et al. Obesity and the risk of heart failure. N Engl J Med 2002;347:305-13. |
|
5.
|
Peterson LR, Waggoner AD, Schechtman KB, Meyer T, Gropler RJ, Barzilai B, et al. Alterations in left ventricular structure and function in young healthy obese women: assessment by echocardiography and tissue Doppler imaging. J Am Coll Cardiol 2004;43:1399-404. |
|
6.
|
Gustafsson F, Kragelund CB, Torp-Pedersen C, Seibaek M, Burchardt H, Akkan D, et al. Effect of obesity and being overweight on long-term mortality in congestive heart failure: influence of left ventricular systolic function. Eur Heart J 2005;26:58-64. |
|
7.
|
Wong CY, O’Moore-Sullivan T, Leano R, Byrne N, Beller E, Marwick TH. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation 2004;110:3081-7. |
|
8.
|
Grandi AM, Maresca AM, Giudici E, Laurita E, Marchesi C, Solbiati F, et al. Metabolic syndrome and morphofunctional characteristics of the left ventricle in clinically hypertensive nondiabetic subjects. Am J Hypertens 2006;19:199-205. |
|
9.
|
Sasso FC, Carbonara O, Nasti R, Marfella R, Esposito K, Rambaldi P, et al. Effects of insulin on left ventricular function during dynamic exercise in overweight and obese subjects. Eur Heart J 2005;26:1205-12. |
|
10.
|
Pascual M, Pascual DA, Soria F, Vicente T, Hern?ndez AM, T?bar FJ, et al. Effects of isolated obesity on systolic and diastolic left ventricular function. Heart 2003;89:1152-6. |
|
11.
|
Iacobellis G, Ribaudo MC, Leto G, Zappaterreno A, Vecci E, Di Mario U, et al. Influence of excess fat on cardiac morphology and function: study in uncomplicated obesity. Obes Res 2002;10:767-73. |
|
12.
|
Trovato GM, Catalano D, Caruso G, Squatrito R, Venturino M, Degano C, et al. Relationship between cardiac function and insulin resistance in obese patients. Diabetes Nutr Metab 2001;14:325-8. |
|
13.
|
Lee WY, Park JS, Noh SY, Rhee EJ, Kim SW, Zimmet PZ. Prevalence of the metabolic syndrome among 40,698 Korean metropolitan subjects. Diabetes Res Clin Pract 2004;65:143-9. |
|
14.
|
World Health Organization, International Association for the Study of Obesity, International Obesity Taskforce. The Asia-Pacific perspective: redefining obesity and its treatment. Sydney: Health Communications; 2000. |
|
15.
|
Quinones MA, Waggoner AD, Reduto LA, Nelson JG, Young JB, Winters WL Jr, et al. A new, simplified and accurate method for determining ejection fraction with two-dimensional echocardiography. Circulation 1981; 64:744-53. |
|
16.
|
Ha JW, Oh JK, Pellikka PA, Ommen SR, Stussy VL, Bailey KR, et al. Diastolic stress echocardiography: a novel noninvasive diagnostic test for diastolic dysfunction using supine bicycle exercise Doppler echocardiography. J Am Soc Echocardiogr 2005;18:63-8. |
|
17.
|
Wong CY, O’Moore-Sullivan T, Fang ZY, Haluska B, Leano R, Marwick TH. Myocardial and vascular dysfunction and exercise capacity in the metabolic syndrome. Am J Cardiol 2005;96:1686-91. |
|
18.
|
Sundstr?m J, Lind L, Nystr?m N, Zethelius B, Andr?n B, Hales CN, et al. Left ventricular concentric remodeling rather than left ventricular hypertrophy is related to the insulin resistance syndrome in elderly men. Circulation 2000;101:2595-600. |
|
19.
|
Paolisso G, Galderisi M, Tagliamonte MR, de Divitis M, Galzerano D, Petrocelli A, et al. Myocardial wall thickness and left ventricular geometry in hypertensives. Relationship with insulin. Am J Hypertens 1997;10:1250-6. |
|
20.
|
Cuspidi C, Meani S, Fusi V, Severgnini B, Valerio C, Catini E, et al. Metabolic syndrome and target organ damage in untreated essential hypertensives. J Hypertens 2004;22:1991-8. |
|
21.
|
Burchfiel CM, Skelton TN, Andrew ME, Garrison RJ, Arnett DK, Jones DW, et al. Metabolic syndrome and echocardiographic left ventricular mass in blacks: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation 2005;112:819-27. |
|
22.
|
Palmieri V, Bella JN, Arnett DK, Liu JE, Oberman A, Schuck MY, et al. Effect of type 2 diabetes mellitus on left ventricular geometry and systolic function in hypertensive subjects: Hypertension Genetic Epidemiology Network (HyperGEN) study. Circulation 2001;103:102-7. |
|
23.
|
Milani RV, Lavie CJ, Ventura HO, Kurtz J, Messerli FH. Effect of left ventricular remodeling on mortality in 35,602 patients with normal systolic function. J Am Coll Cardiol 2003;41:459A. |
|
24.
|
Brutsaert DL, De Keulenaer GW. Diastolic heart failure: a myth. Curr Opin Cardiol 2006;21:240-8. |
|
25.
|
Aurigemma GP, Silver KH, Priest MA, Gaasch WH. Geometric changes allow normal ejection fraction despite depressed myocardial shortening in hypertensive left ventricular hypertrophy. J Am Coll Cardiol 1995;26: 195-202. |
|
26.
|
Vinereanu D, Nicolaides E, Tweddel AC, Fraser AG. “Pure” diastolic dysfunction is associated with long-axis systolic dysfunction. Implications for the diagnosis and classification of heart failure. Eur J Heart Fail 2005; 7:820-8. |
|
27.
|
Jones CJ, Raposo L, Gibson DG. Functional importance of the long axis dynamics of the human left ventricle. Br Heart J 1990;63:215-20. |
|
28.
|
Derumeaux G, Ovize M, Loufoua J, Andr?-Fouet X, Minaire Y, Cribier A, et al. Doppler tissue imaging quantitates regional wall motion during myocardial ischemia and reperfusion. Circulation 1998;97:1970-7. |
|
29.
|
Nikitin NP, Witte KK, Thackray SD, de Silva R, Clark AL, Cleland JG. Longitudinal ventricular function: normal values of atrioventricular annular and myocardial velocities measured with quantitative two-dimensional color Doppler tissue imaging. J Am Soc Echocardiogr 2003;16:906-21. |
|
30.
|
Al Suwaidi J, Higano ST, Holmes DR Jr, Lennon R, Lerman A. Obesity is independently associated with coronary endothelial dysfunction in patients with normal or mildly diseased coronary arteries. J Am Coll Cardiol 2001;37:1523-8. |
|
31.
|
Turhan H, Erbay AR, Yasar AS, Bicer A, Sasmaz H, Yetkin E. Impaired coronary blood flow in patients with metabolic syndrome: documented by Thrombolysis in Myocardial Infarction (TIMI) frame count method. Am Heart J 2004;148:789-94. |
|
32.
|
Engeli S, Negrel R, Sharma AM. Physiology and pathophysiology of the adipose tissue renin-angiotensin system. Hypertension 2000;35:1270-7. |
|
|
|