INTRODUCTION
Early gastric cancer (EGC) is defined as a lesion confined to the mucosa or the submucosal layer, regardless of metastasis to the lymph node. The prognosis of EGC is well good, with a 5-year survival rate of over 90% after radical gastrectomy [1]. In general, it is believed that untreated EGC progresses to advanced gastric cancer (AGC), but it is not clear whether all EGCs progress to AGC [2]. Understanding natural history of EGC is essential to accurately determine the duration, prognosis, treatment option, and timing of treatment in certain circumstances that require treatment delay. The critical factor when evaluating the advantage and disadvantages of endoscopic or surgical treatment is the natural history of cancer. However, there are few studies on the natural history of gastric cancer, especially EGC, to date, because patients refusing endoscopic or surgical treatment after the diagnosis of EGC are rare in the clinic. We report a rare case of natural history in an EGC patient who refused cancer treatment and occurred cancer-related perforation and death later.
CASE REPORT
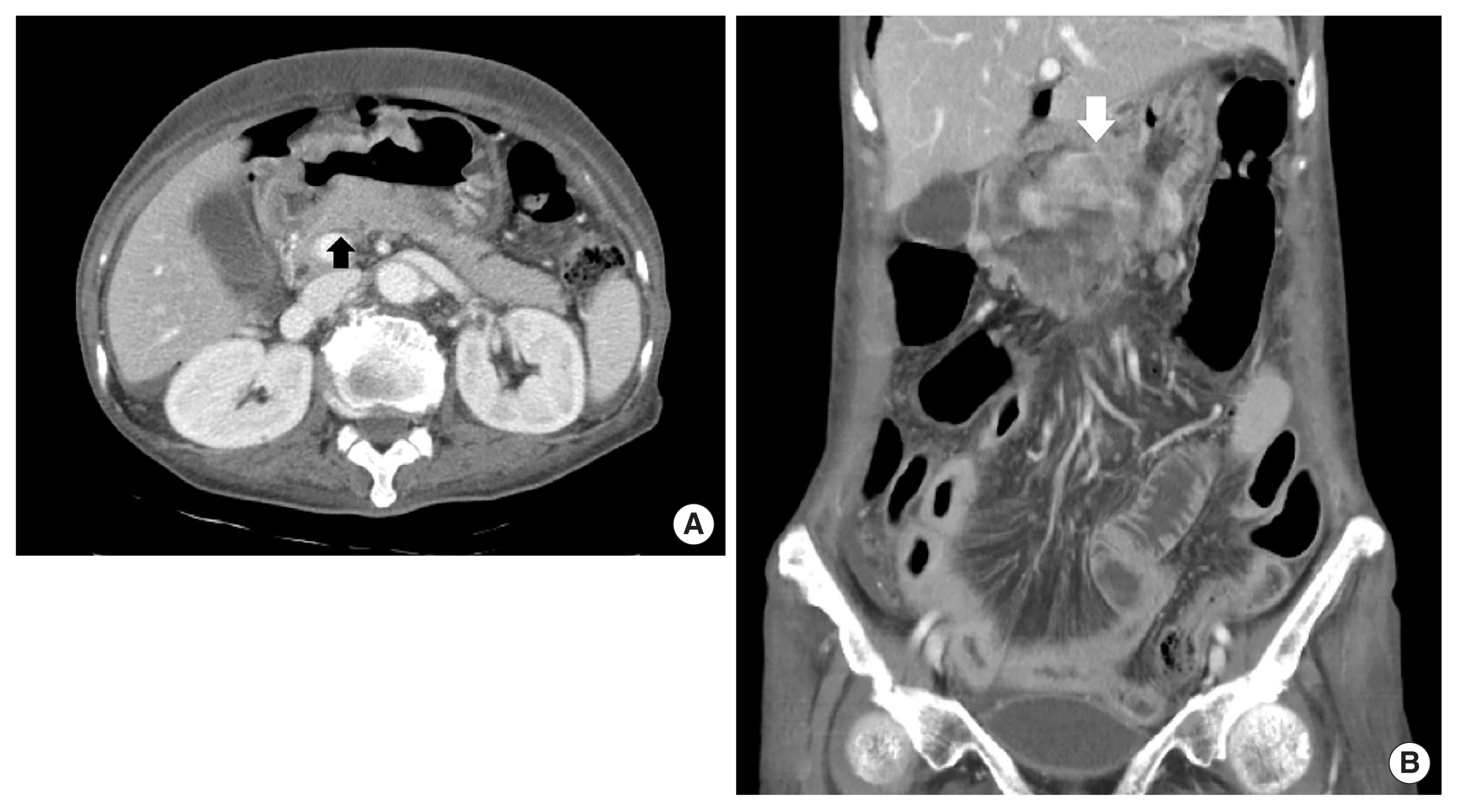
A 79-year-old female patient complaining of intermittent epigastric pain received a regular medical checkup with esophagogastroduodenoscopy at a local hospital. A shallow depressed lesion with redness was observed at the lesser curvature of the prepyloric antrum, and type-IIc EGC was diagnosed. Biopsy revealed signet-ring cell adenocarcinoma (SRC). The patient was referred to Soonchunhyang University Bucheon Hospital for evaluation of gastric cancer. We performed an endoscopic ultrasound and confirmed type IIc EGC with the invasion of the submucosal layer of the stomach (Fig. 1). The patient without family history had been diagnosed with severe asthma and bronchiectasis 30 years earlier. Abdominal computed tomography (CT) with contrast showed a thickened wall at the lesser curvature of the antrum in the stomach without lymph node enlargement (Fig. 2). There were severe obstructive and restrictive ventilatory defects in the pulmonary function test. We recommended endoscopic treatment for EGC, but she refused any intensive treatment due to her old age and comorbidity disease and was subsequently discharged. After the hospital discharge, the patient received palliative treatment such as antacid, digester, and painkillers to relieve heartburn and abdominal discomfort.
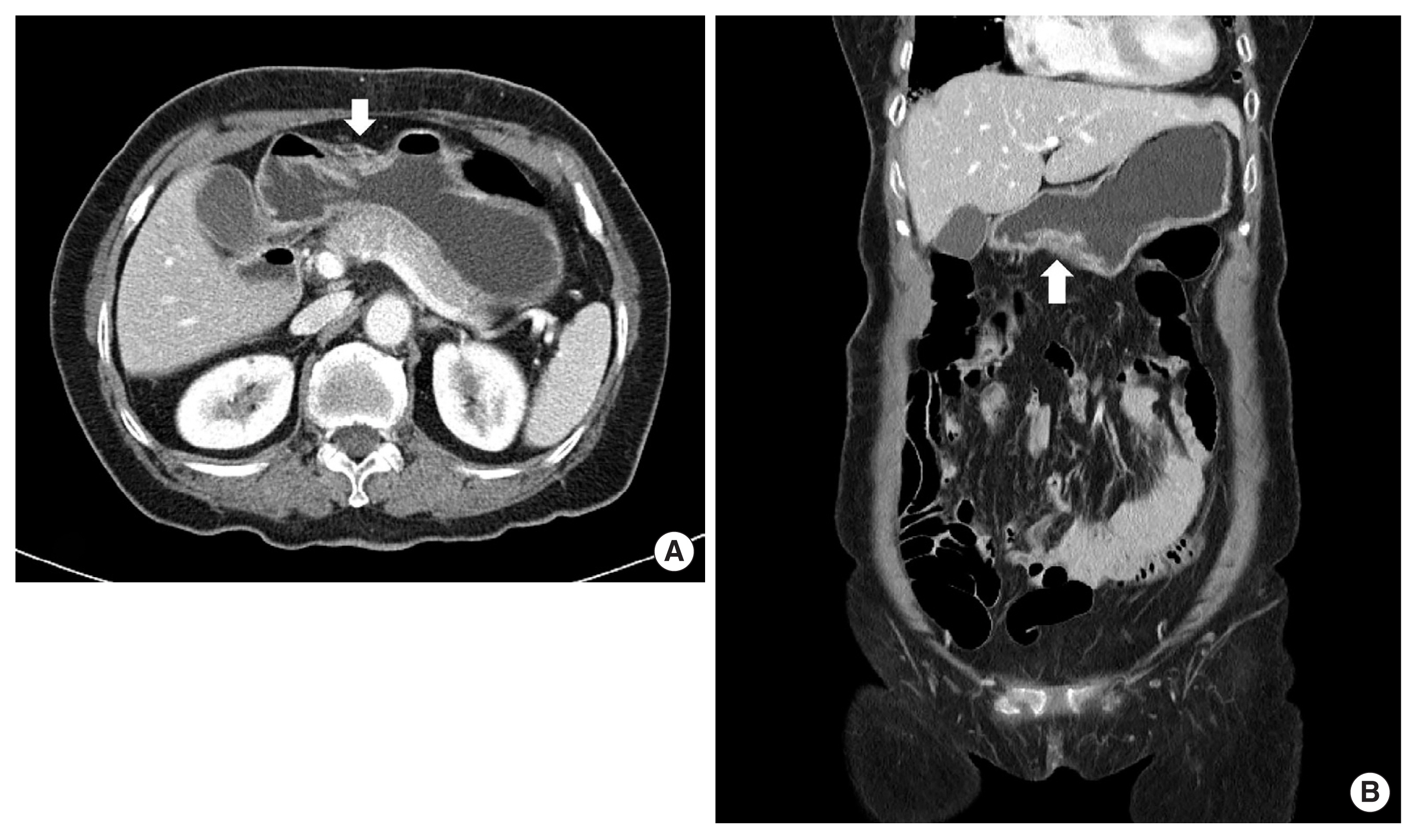
Twenty-nine months after the initial diagnosis, the patient intermittently developed a small amount of hematochezia and hematemesis. Due to the aggravation of hematemesis and severe abdominal pain 6 days later, she was admitted to the emergency department of our hospital. Upon arrival at our hospital, her blood pressure was 90/60 mm Hg, heart rate was 110 beats/min, respiratory rate was 28 breaths/min, body temperature was 37.8°C, and SpO2 was 93% with O2 5 L inhalation with a nasal cannula. Physical examination revealed abdominal distension with and tenderness and rebound tenderness on the whole abdomen. The laboratory data were as follows: hemoglobin 6.1 g/dL, white blood cell count 26,870/μL (segment neutrophils 94%), platelet count 512,000/μL, blood urea nitrogen 13.3 mg/dL, creatinine 0.6 mg/dL, and high-sensitivity C-reactive protein 9.51 mg/dL. Abdominal CT with contrast showed AGC with direct invasion to the pancreas and pneumoperitoneum due to cancer-related perforation of the stomach (Fig. 3). For emergency laparotomy, we transferred her to an operation room, and the anesthesiologist performed general anesthesia on her. After mechanical ventilation, while PaO2 was within the normal range as 153 mm Hg in FiO2 50%, PaCO2 was abruptly increased as 91.4 mm Hg despite adequate ventilation, resulting in respiratory acidosis as PH 7.105. Thus, we had to cancel the emergency laparotomy, and the patient was received conservative treatment with the administration of intravenous antibiotics. Fortunately, the patient’s condition steadily improved. On hospital day 10, there was no complication after a progression of the diet. The patient was discharged on hospital day 15. The patient provided written informed consent for the publication of clinical details and images.
After discharge, she continued palliative treatment, including painkiller, oral antibiotics, and antacid. Forty months after the initial diagnosis, the patient was transferred to the emergency department after a large amount of hematemesis. On arrival, vital signs included a blood pressure of 70/40 mm Hg, a pulse of 130 beats/min, a respiratory rate of 26 breaths/min, and a body temperature of 38.3°C. We conducted a limited physical examination because of her drowsy mental status but was at least able to note the patient’s abdomen with muscle guarding. Arterial blood gas analysis revealed that the patient had severe metabolic acidosis (PH 6.98, PaCO2 53 mm Hg, PaO2 50 mm Hg, HCO3− 10.8 mmol/L). The laboratory data upon arrival were as follows: hemoglobin 4.7 g/dL, white blood cell count 21,230/μL (segment neutrophils 90%), platelet count 23,000/μL, blood urea nitrogen 32.9 mg/dL, creatinine 2.1 mg/dL, and high-sensitivity C-reactive protein 42.75 mg/dL. Abdominal CT showed repeated cancer-related perforation in the stomach, accompanied by cancer peritonei (Fig. 4). Despite aggressive fluid therapy with broad-spectrum antibiotics in the intensive care unit, the patient died from septic shock on hospital day 5.
DISCUSSION
Compared to AGC, the 5-year survival rate and recurrence rate of EGC received appropriate treatment are significantly better as 90% and more and 1.0%–11.2%, respectively [3]. Because of the excellent prognosis of EGC, almost all patients diagnosed with EGC receive an endoscopic intervention, such as endoscopic mucosal resection, endoscopic submucosal dissection, or surgical resection of the stomach. Thus, little is known about the course of untreated EGC, and there are few studies about the natural history of EGC. EGC has a relatively long natural course compared to AGC. EGC, like benign gastric ulcers, is known to have a life cycle that repeats the development and healing of the ulcer. Tsukuma et al. [2] investigated 56 patients diagnosed with EGC for 10 years. During the mean follow-up period of 39 months (range, 6–137 months), 20 patients are remained in the early stage, while 36 patients (64.3%) progressed to the advanced stage. The median duration of those who remained in the early stage was 44 months, and the proportion remaining in the early stage steadily declined with time. Jeong et al. [4] also investigated 12 patients with untreated EGC for 10 years via endoscopy follow-up. During the mean follow-up period of 25 months, three patients (25%) diagnosed with EGC progressed to AGC, and the overall 5-year survival rate was estimated at 45%. Based on these results, EGC is a precursor to the development of AGC, and untreated EGCs eventually progress to AGC with time. In the present case, type IIc EGC progressed to advanced cancer at 29 months and rapidly grew to cancer peritonei at 40 months.
The progression time from early to AGC varies, but factors influencing the growth speed and progression remain unclear. Fujisaki et al. [5] reported that progression from mucosal to submucosal cancer took 7 years, and progression from submucosal to AGC took 2–3 years. While the growth speed of small EGC is a slow, large size of EGC, especially exceeding 4 cm in size, typically enlarges rapidly due to an increased proportion of the lesion with deep invasion according to cancer size. Tsukuma et al. [2] showed that the time interval required for half of the patients with EGC under the age of 75 years to progress AGC is 50 months, while those over the age of 75 years have 36 months. In the present case, the patient has been initially diagnosed with a type IIc EGC with an invasion of the submucosal layer accompanied by without lymph node metastasis. It is unclear when an intramucosal lesion first appeared and infiltrated the submucosal layer, but AGC was confirmed at the second hospitalization 29 months after the initial diagnosis. This finding is consistent with the result of the study of Tsukuma et al. [2], which showed rapid progression time in older patients over the age of 75 years. AGC with SRC showed a similar or higher rate of lymph node metastasis than other types of carcinoma and diffuse infiltration of the gastric wall, leading to a higher rate of peritoneal dissemination than non-SRC [6]. On the other hand, recent studies for SRC of EGC reported a favorable prognosis due to a lower rate of lymph node metastasis and a higher prevalence of mucosa-confined lesions in SRC [7]. In the present case, however, we could not perform repetitive endoscopy to investigate the progression process of the type IIc EGC due to her refusal of invasive treatment and follow-up loss.
Old patients with EGC sometimes refuse treatment. Among these, many patients have given up further treatment when cancer-related complications occur, particularly after implementing the law as the withdrawal of life-sustaining treatment. In addition, the development of endoscopic devices leads to earlier detection and diagnosis with high sensitivity and specificity for superficial gastric malignancies. Moreover, the rapid enhancement of the endoscopic resection method, including endoscopic mucosal resection and endoscopic submucosal dissection, is related to a high curative rate comparable with those of traditional gastrectomy and makes it easier for the patient to receive treatment [8]. So, it will be more challenging to investigate the natural history of untreated EGC. Although surgical treatment of elderly patients with gastric cancer contributes to reasonable long-term survival rates, recommending surgery in elderly patients with less functional capacities and comorbidities is difficult. The most important factors when evaluating surgical treatment’s advantages and disadvantages are life expectancy and the natural history of gastric cancer. However, there are few studies on the natural history of gastric cancer to date. This case may help to understand the natural progression of untreated EGC. Thus, we report a case of natural history in an untreated EGC patient that progressed to advanced cancer, leading to repeated gastric perforation and cancer-related death.