INTRODUCTION
The clinical spectrum of hepatitis A virus (HAV) infection ranges from asymptomatic infection to fulminant hepatitis [1]. The cases of post-hepatitis aplastic anemia (AA) following HAV infection have been very rarely reported. Although no correlations have been observed between severity of hepatitis and AA, if it happens once, the bone marrow (BM) failure can be severe and the mean survival rate after developing severe BM aplasia has been 2 months. Moreover, it is usually fatal if untreated, and the fatality rate ranges from 78% to 88% [2].
Hepatitis-associated aplastic anemia (HAA) may be induced by hepatitis B or C virus infection or by infections with other viruses, such as human immunodeficiency virus (HIV), Epstein-Barr virus, transfusion-transmitted virus and echovirus [3]. However, the etiological agent responsible for most cases of post-hepatic AA remains unknown [4]. To our knowledge the only two cases of hepatitis A-associated aplastic anemia (HAAA) have been reported so far [5,6]. Those were the case of a 6-year-old boy which was first reported in 1978 [5] and a case of 27-year-old Japanese woman to be diagnosed by radioimmunoassay of immunoglobulin M (IgM) anti HAV for the first time in 1987 [6]. In the first case, pancytopenia was developed simultaneously with hepatitis and the marrow transplant was postponed because of the possibility that high doses of cyclophosphamide might not be well tolerated in the presence of active hepatic disease. The patient’s clinical status gradually improved with only conservative care. In case of second patient, AA was diagnosed 7 weeks after the development of hepatitis and the patient was treated with anabolic steroid therapy (metenolone enanthate) and pulse therapy (methyl prednisolone). However, there has been no other report treated with bone marrow transplantation (BMT) so far. Considering that the HAA is a rare disease but the prognosis is poor without early diagnosis and early treatment, the suspicion of HAAA can be pivotal. Herein, we report the case of AA following severe HAV infection, and would like to focus on this very rare, but clinically important disorder.
CASE REPORT
A previously healthy 12-year-old boy presented with jaundice for two days. There was no history of exposure to drug with no prior episode. He was alert and the vital sign was stable. The physical examination presents icteric sclera, hepatomegaly, and mild abdominal tenderness without rebound tenderness, but showed no bleeding sign.
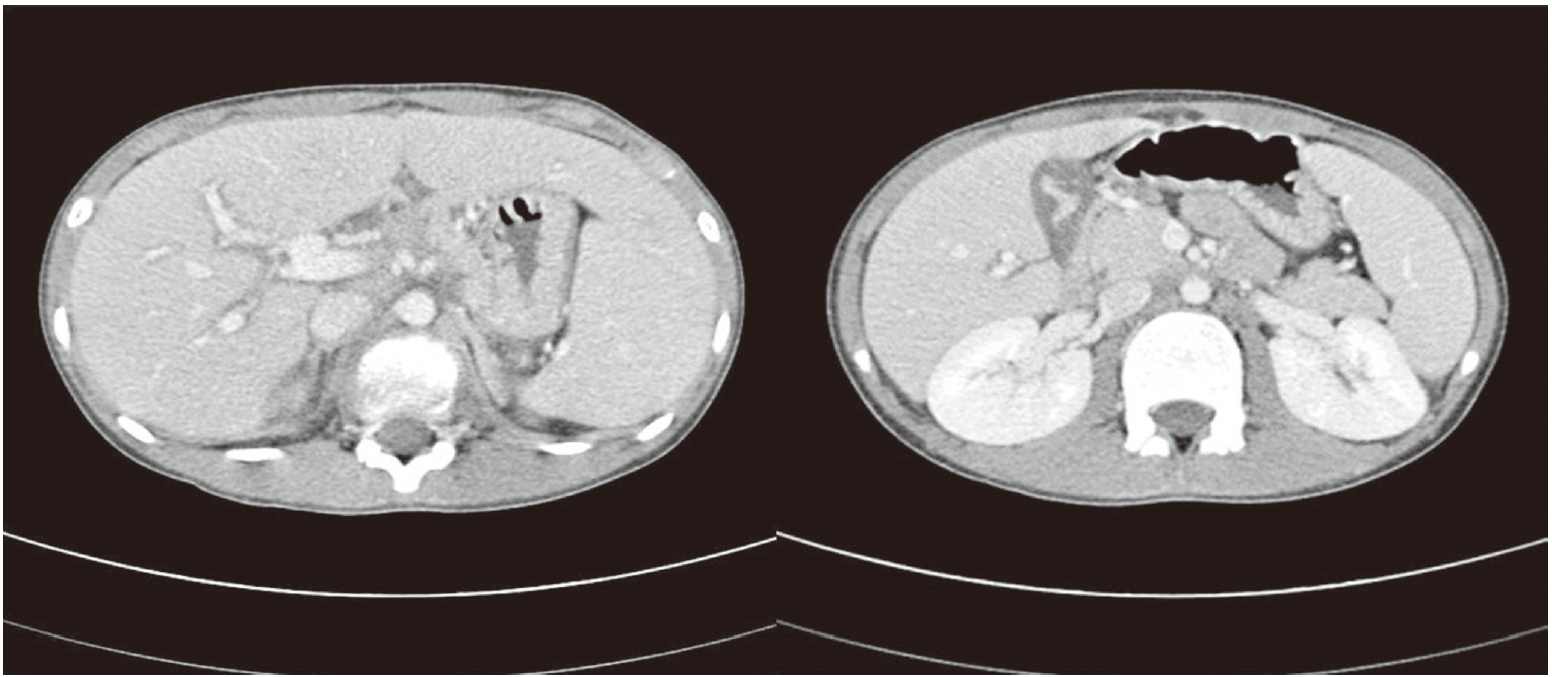
The laboratory findings on admission were as follows: leukocyte count 5,060/mm³ with 42% neutrophils; hemoglobin 13.6 g/dL; platelet count 300×10³/mm³; blood urea nitrogen 7.2 mg/dL; creatinine 0.54 mg/dL; total protein 6.6 g/dL; albumin 3.9 mg/dL; aspartate aminotransferase (AST)/alanine aminotransferase (ALT) 1,060/1,377 IU/L; total bilirubin 9.9 mg/dL; glucose 117 mg/dL; prothrombin time 68% (international normalized ratio [INR], 1.3); and activated partial thromboplastin time 36.5 seconds. Serologic tests for hepatitis B, C, Epstein-Barr virus, cytomegalovirus, parvovirus B19, and HIV were all negative, whereas IgM anti HAV was positive. Abdominal computerized tomography revealed hepatosplenomegaly, periportal edema, edematous change of gallbladder, and small lymphadenopathy in left gastric and portocaval space (Fig. 1).
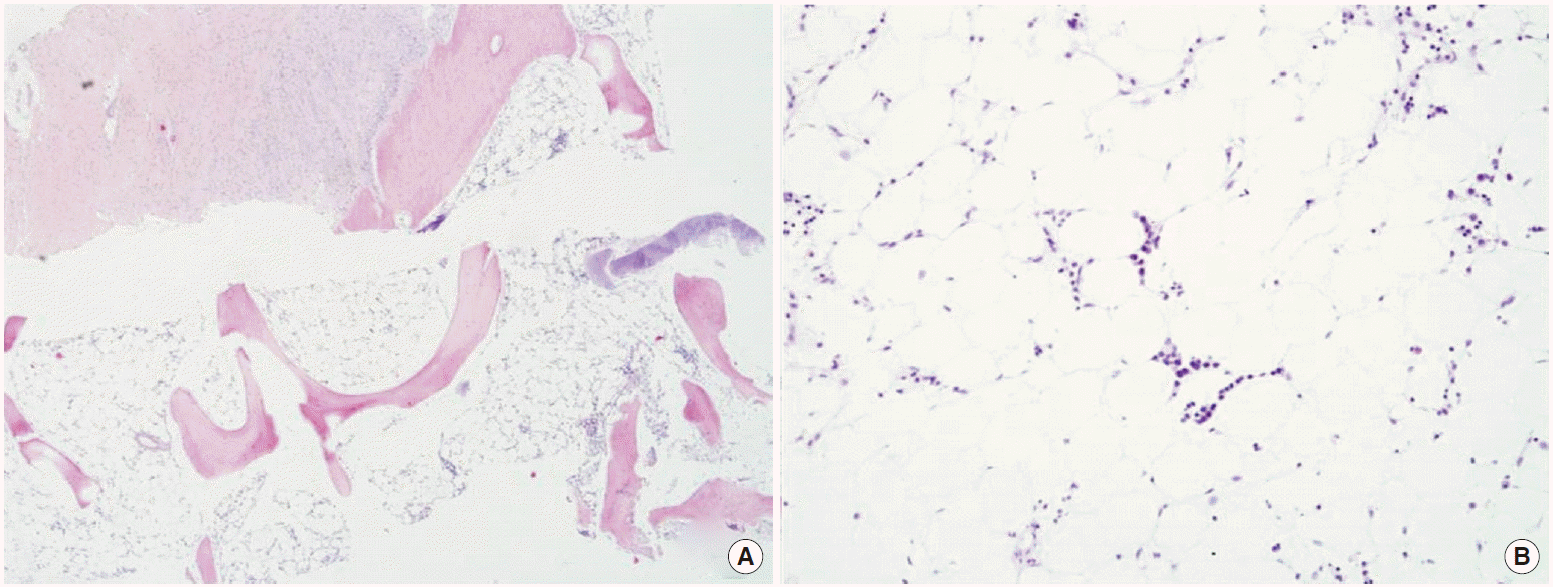
On the twentieth day after admission, the liver function was improved as following: AST 56 IU/L, ALT 86 IU/L, total bilirubin 1.2 mg/dL, and INR 0.94, but pancytopenia developed; absolute neutrophil count fell to 1,098/μL, hemoglobin 11.8 g/dL, and platelet count 53×10³/mm³. Peripheral blood showed anisocytosis, leucopenia, thrombocytopenia. Direct/indirect Coomb’s tests proved negative. The levels of vitamin B12 and folates were within a normal range. BM examination showed hypoplasia with a low cellularity of less than 10%. Megakaryocytes are markedly decreased in number. On touch print preparations, hematopoietic cells are decreased in number (Fig. 2). The patient was consequently diagnosed with HAAA.
Then, he was administered anti-thyroglobulin at a dose of 4 mg/kg for 6 days, methylprednisolone at 2 mg/kg/day intravenously for 10 days, and oral cyclosporine at 4 mg/kg per day orally, which adjusted trough levels to around 250 ng/mL. Granulocyte colony-stimulating factor was administered for 8 days. Although liver enzymes and serum bilirubin levels returned to normal by the five week of admission, no hematological response to treatment followed. Pancytopenia was persisted, meeting the criteria for very severe AA, which led to BMT at forty days after initiating BM failure. After successful BMT, the patient was recovered and discharged.
DISCUSSION
HAA is a rare variant of AA, in which marrow failure follows the development of hepatitis. It has been reported in 2% to 5% of cases in the West, and in 4% to 10% of cases in the Far East [7]. Although HAA is not considered to be related to age, sex, and severity of hepatitis, it has been predominantly found in children, adolescent boys, and in young aged men [7] who develop severe pancytopenia 2 to 3 months after an episode of acute hepatitis. However, the etiological agent responsible for most cases of HAA remains unknown [4]. Even in case of HAAA, only two cases have been reported in the literature [5,6].
HAA is considered to have three different mechanisms. One is the direct suppression of BM by the hepatitis virus, the second is the failure of the damaged liver, and last one is the immunological mechanisms [8]. Some study results explain an immune-mediated pathogenesis. Two of the possible mechanisms of HAA are T-cell mediated suppression of BM and liver infiltration by activated CD8 cells [9,10]. Auto-reactive T-lymphocytes from the BM of patients with AA have been shown to inhibit hematopoiesis. Interferon (IFN)-c gene expression is prevalent in the BM of patients with acquired AA. Thus, BM inhibition may be mediated by the release of IFN-c, a marrow suppressing cytokine [11]. It is implicated that viruses induce lymphocyte activation and ultimately apoptotic death of hematopoietic cells in the BM. Meanwhile, the time interval between the occurrence of hepatitis and the onset of BM failure in HAA suggests that the initial target organ of the immunological response is the liver. Viral infections induce lymphocytic infiltrate in the liver at the time of maximal increases in serum ALT levels [4]. Liver histology is characterized by T cell infiltrating the parenchyma as reported in acute hepatitis [12].
Even though acute HAV infection generally follows a benign clinical course, showing a partial or complete resolution [9], most of the clinical features relating to AA following the hepatitis include pallor, multiple skin bleeding, lymphocytopenia, hypogammaglobulin, neutropenia, or fever [7]. In this case, the patient presented with jaundice, and pancytopenia was noted on twenty days after the diagnosis of acute HAV infection. However, there was no bleeding and secondary infection.
The BM failure can be severe and it is usually fatal if untreated, no correlations have been observed between severity of hepatitis and AA. Since HAA could be a life-threatening condition [2], its therapeutic approach should be considered as a medical emergency. First-line treatment is allogeneic hematopoietic stem cell transplantation from human leukocyt antigen-matched sibling donor (MSD). In patients without an available MSD, immunosuppressive therapy with cyclosporine and antithymocyte immunoglobulin is recommended. Transplantation of marrow from an alternative donor is indicated as a salvage therapy for non-responders to the first regimen of immunosuppressive therapy [13].
In this case, the patient was received immunosuppressive therapy with antithymocyte immunoglobulin for the initial treatment. However, since the marrow failure had been progressed despite the therapy, hematopoietic stem cell transplantation was performed and the patient got recovered after BMT. It is not only the rare case of AA occurring during the course of acute HAV infection, but also the first report of patient with severe AA required BMT for HAAA.
Generally, severe pancytopenia develops 2 to 3 months after an episode of acute hepatitis. Likewise, in this case, the initial complete blood count at admission was within normal limit and pancytopenia was noted on twenty days after the diagnosis of acute HAV infection. Thus, even if there was no evidence of pancytopenia at diagnosis or at the beginning of treatment, the patient with acute HAV infection should be monitored in order not to miss the progression of pancytopenia.
In conclusion, based on this case, we highlight the importance of suspicion of HAAA when pancytopenia is developed even in patient with HAV infection. Especially, if the pancytopenia persists, HAAA should be considered as differential diagnosis, and peripheral blood smear should be performed.