Anesthetic Management of Emergency Surgery for a Patient with Vascular Ehlers Danlos Syndrome (Type IV): A Case Report
Article information
Abstract
Ehlers-Danlos syndrome (EDS) is a very rare genetic disorder characterized by defects in the production of connective tissue. Among them, vascular EDS is the subtype known to have the worst prognosis due to fragile blood vessels. Accordingly, we would like to report a case report of a patient with vascular EDS with a review of the literature on possible massive transfusion and anesthesiological problems. Patients with vascular EDS have very weak blood vessels and tissues that are easily broken. So there is a high possibility of unexpected massive bleeding during emergency surgery in these patients. Therefore, the anesthesiologist should be fully prepared for the possibility of massive blood loss, keeping in mind the possibility of damage to large blood vessels. The central vein must be secured using ultrasound, sufficient blood and fluids must be prepared, and equipment capable of rapid administration must be perfectly prepared before the start of operation.
INTRODUCTION
Ehlers-Danlos syndrome (EDS) is a genetic disorder characterized by defects in connective tissue production. EDS has several subtypes. Among them, vascular EDS is known to have the worst prognosis because massive hemorrhage due to vascular weakness or aneurysm appears at the age of about 40 years [1,2].
When performing emergency surgery for patients with vascular EDS, the current patient’s bleeding amount and bleeding tendency as well as the possibility of bleeding from the other than surgical site should be identified. The anesthesiologist should use ultrasound to prevent additional damage to other blood vessels when securing the central venous line. Sufficient blood should be prepared with the possibility of massive bleeding in mind, and surgery should not be performed before setting up a machine that can quickly transfuse blood and fluids.
Patients with vascular EDS are generally very rare to contact anesthesiologists. Unlike other patients with massive bleeding, we wanted to raise awareness of the possibility of additional massive bleeding in addition to radiological findings. The purpose of this study is to describe the points that must be considered when rapidly preparing for emergency surgery for such patients.
CASE REPORT
The patient, a 38-year-old male, visited a nearby hospital complaining of loss of consciousness and pallor. Damage to the superior mesenteric artery (SMA) branch and hemoperitoneum were observed on abdominal computed tomography (CT) scan, so the patient was transferred to Hanyang University Guri Hospital for an emergency surgery.
The patient had a history of vascular EDS diagnosed in 2015. The patient’s mother was also diagnosed with EDS, and had a history of sudden death from cerebral hemorrhage in middle age.
Upon arrival at the emergency room, on physical examination, the vital signs were as follows: body temperature, 35.0°C; blood pressure, 130/110 mm Hg; and heart rate, 123 beats/min. After 4 minutes, oxygen saturation was confirmed to be 80%. Five minutes after arriving at the emergency room, the patient suffered cardiac arrest, and after 2 minutes of advanced cardiovascular life support, a return of spontaneous circulation (ROSC) was achieved. After that, tracheal intubation and ventilator support were performed. the vital signs were as follows: body temperature, 35.0°C; blood pressure, 90/71 mm Hg; heart rate, 63 beats/min; and oxygen saturation (SpO2) 85%.
Laboratory results performed after ROSC in the emergency room included white blood cell count of 17.1 thousand/mm3, hemoglobin of 7.5 g/dL, hematocrit of 24.5%, platelet count of 104 thousand/mm3, prothrombin time (%) of 44%, and international normalized ratio of 1.88. When he was intubated, arterial blood gas analysis (ABGA) results included pH of 6.794, pCO2 (partial pressure of carbon dioxide) of 49.6 mm Hg, pO2 (partial pressure of oxygen) of 59.7 mm Hg, HCO3- of 7.4 mmol/L, and base excess −27.2 mmol/L (Table 1).
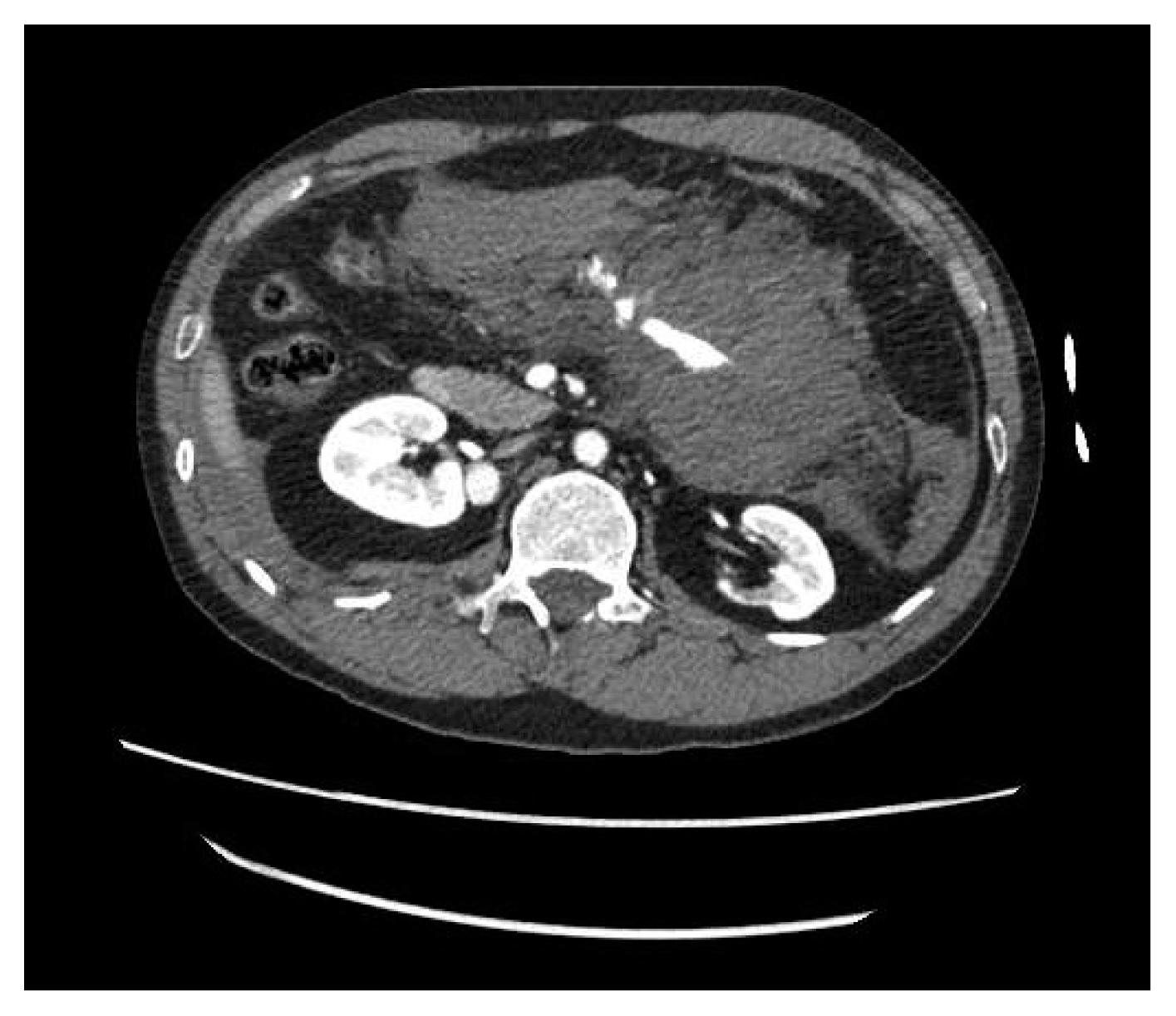
There are abdominopelvic cavity hematoma and hemoperitoneum on radiological examination performed at the private clinic that was first visited (Fig. 1). And there is a SMA branch active bleeding (Fig. 2). And we also found another vascular problem. There are both renal aneurysms (Fig. 3) and splenic artery aneurysm (Fig. 4). After being transferred to our hospital, no additional preoperative radiological examination was performed.

Preoperative axial image from computed tomography with precontrast. High density fluid collection and infiltration in abdominopelvic cavity.
Preoperative axial image from computed tomography (CT) with portal phase contrast CT. In the branches of the superior mesenteric artery, leakage of contrast material, which appears as active bleeding, is observed.

Preoperative axial image from computed tomography (CT) with contrast CT. Bilateral renal artery aneurysm is observed.

Preoperative axial image from computed tomography (CT) with contrast CT. Splenic artery aneurysm is observed.
A central venous passage was secured through the right subclavian vein in the emergency room before surgery, and arterial line was inserted into the left radial artery. Since the procedures were performed in the emergency room, information on the use of ultrasound was unknown. An 18G intravenous line was secured in both upper arms, and foley catheter and tracheal intubation (7.5 mm) were in place. Vital signs at this time were as follows: body temperature, 35.0°C; blood pressure, 75/45 mm Hg; heart rate, 80 beats/min; and SpO2 95%. And the mental status was stupor. Four units of red blood cells were transfused, and sodium bicarbonate 100 mEq and intravenous fluids were administered. Additionally, norepinephrine infusion was maintained at 0.16 mcg/kg/min.
A rapid infusion system was placed in the central venous catheter prior to the start of surgery. Vascular monitoring and norepinephrine and epinephrine infusions were prepared. In addition, the operation was started while administering 2 packs of red blood cells. Anesthesia was induced by injecting rocuronium 50 mg intravenous and remifentanil (40 mcg/mL), and SEDLine (Masimo Corp., Irvine, CA, USA) brain functioning monitoring was performed. Anesthesia was maintained while adjusting sevoflurane from 0 to 0.5 vol% according to the patient’s condition along with FiO2 (fraction of inspired oxygen) 50% air. The airway peak pressure was 31 cmH2O before laparotomy, but it was maintained at 15–18 cmH2O after laparotomy. In addition, norepinephrine infusion at a dose of 0.35 to 0.70 mcg/kg/min and epinephrine infusion at a dose of 0.10 to 0.35 mcg/kg/min were administered to maintain blood pressure. ABGA was performed intraoperatively (Table 1).
Sodium bicarbonate 100 mEq and short-acting insulin 5 IU were administered to correct acidity and blood glucose values in blood tests. And 8 red blood cells and 4 fresh frozen plasma were administered. As for the surgical findings, about 3,000 mL of bleeding was confirmed immediately after laparotomy. The mesentery of the small and large intestine was very easily broken, and bleeding was observed in several places. After ligation of the SMA root, blood pressure temporarily stabilized, but multiple bleeding was still confirmed in the small intestine. Eventually, the surgeon could not control all the bleeding focus and ended the operation after packing 25 pieces of gauze into the abdominal cavity. ABGA was performed before leaving the room (Table 1). The patient was transferred to the intensive care unit while performing ambu-bagging, and he was administered with norepinephrine infusion (0.35 mcg/kg/min) and epinephrine (0.30 mcg/kg/min) while transfusing 1 red blood cell. As it was, a body temperature of 35.5°C, blood pressure of 90/60 mm Hg, and a heart rate of 102 beats/min were measured.
After the patient was transferred to the intensive care unit, the use of inotropes and vasopressor was continued and blood transfusions were performed, but the patient could not keep up with the amount of blood loss and acidosis worsened. On the following day after surgery, cardiac arrest occurred in the afternoon due to continuous bleeding, and he expired. The patient’s caregiver provided written informed consent for the publication of clinical details and images.
DISCUSSION
EDS is a hereditary connective tissue disease and is one of the very rare diseases that have characteristics such as skin hyperextension, joint hypermobility, and tissue weakness. In the 2017 international classification, it is divided into 13 subclasses [2].
Among all types of EDS, vascular EDS corresponding to this case is one of the worst prognostic types, with an estimated prevalence of about 1/150,000 [3]. Vascular EDS has a very poor prognosis due to the possibility of arterial rupture and organ damage occurring at a relatively young age. Malfait et al. [1] reported that 75% of patients molecularly diagnosed with vascular EDS suffered major complications before the age of 40 years, with the majority (82%) being arterial-related complications. In this case, damage to the branch of the SMA was confirmed. In addition, 15% of patients experienced organ-related complications and most of them are known to be problems with the sigmoid colon [1,4]. In this case, damage to the mesentery of the small and large intestines and bleeding in several parts of the small intestine were observed.
Wiesmann et al. [5] suggested that history taking and physical examination can be performed on preoperative vascular EDS patients in scheduled surgery. In addition, they said that a more detailed examination on the history of bleeding along with a general evaluation should be performed before surgery, and that a genetic judgement could be performed if it is needed. In a situation where emergency surgery is planned, as in this case, it is more important to identify factors that may increase the patient’s risk of bleeding and to prepare for massive bleeding rather than securing the above information. In addition, through the radiological examination results, besides the most problematic part, other radiological findings that may cause bleeding should be identified, and it should be kept in mind that bleeding may occur in other parts of the patient at any time. In this case, no other radiological examination was performed other than abdominal CT. Before surgery, additional radiological examinations could not be performed in order to perform emergency surgery immediately. After surgery, it was decided not to perform additional radiological examinations due to the very low survival rate of the patient.
Berney et al. [6] reported that in patients with vascular EDS, the tissues were very brittle and surgically described as “wet blotting paper.” For this reason, the anesthesiologist should be prepared for the possibility of bleeding and the possibility of expanding the surgical range, and regarding these matters, the selection and scope of the surgical method should be decided through sufficient discussion with the surgeon [6,7]. In this case, the plan for open surgery was discussed with the surgeon prior to surgery. However, the possibility of expanding the surgical range was not predicted. During surgery, the surgeon described the tissue as continuing to tear. Also, the blood vessel was torn continuously even at the ligation site.
Stine and Becton [8] discussed the possibility of using desmopressin acetate for the treatment of bleeding in patients with vascular EDS. Faber et al. [9] claimed that recombinant factor VIIa could be applied to patients with vascular EDS. It was regrettable that aggressive hemostatic therapy was not considered during surgery in this case. The use of hemostatic treatment after surgery was not confirmed.
Bouaziz et al. [10] strongly recommended the use of ultrasound when securing a central venous catheter in adult patients, and made weak recommendations for securing an arterial catheter and peripheral vein. Considering the condition of tissues and blood vessels of patients with vascular EDS, more careful judgment is required and should be performed carefully when securing the patient’s venous and arterial routes. Wiesmann et al. [5] recommend avoiding securing a central venous catheter and arterial catheter as much as possible, and recommend using ultrasound if necessary. But, in this case, the patient was in a situation where he had to receive a large amount of blood transfusion, and needed drug injection, it was necessary to secure more invasive intravenous lines. In addition, it was determined that invasive blood pressure monitoring was absolutely necessary due to the unstable blood pressure situation. In the case of this patient, all lines were secured in the emergency room, so it was difficult to accurately determine the method used.
For anesthesiologists, EDS is one of the rarest diseases. In particular, vascular EDS has a poor prognosis because massive bleeding can occur due to damage to blood vessels and tissues. In performing surgery under general anesthesia, there are representative risk factors such as the possibility of massive bleeding and bleeding tendency. There is a high possibility that surgery will be performed based on insufficient information about patients diagnosed with vascular EDS and insufficient knowledge about the disease. Therefore, if there is a situation in which emergency surgery for a patient with vascular EDS must be performed urgently, the patient’s current expected bleeding amount and bleeding tendency should be first identified. In addition, it is necessary to predict the possibility of additional vascular problems in addition to the judgment on the condition of the patient’s blood vessels and organs and the problem area through the radiological examination results. It is important to remember that it cannot be resolved only by ligation of the expected bleeding site. In situations where massive hemorrhage is suspected, central venous access should be secured using ultrasound with caution. In addition, anesthesia and surgery should be started after sufficient blood is prepared and equipment capable of rapid injection of blood and fluid is perfectly prepared. It was regrettable that we did not anticipate the possibility that the bleeding could not be controlled by the surgical method. We need to consider the use of hemostatic therapy as an alternative approach.
Notes
No potential conflict of interest relevant to this article was reported.
