Metastasectomy for Recurrent or Metastatic Biliary Tract Cancers: A Single Center Experience
Article information
Abstract
Objective
Efficacy or long-term result of metastasectomy for recurrent or metastatic biliary tract carcinoma (BTC) is not well established. We conducted a retrospective review of the outcomes of metastasectomy for recurrent or metastatic BTCs.
Methods
The clinicopathological features and outcomes of consecutive patients with BTCs who underwent surgical resection for primary and metastatic disease at a tertiary referral hospital from 2003 to 2013 were reviewed retrospectively.
Results
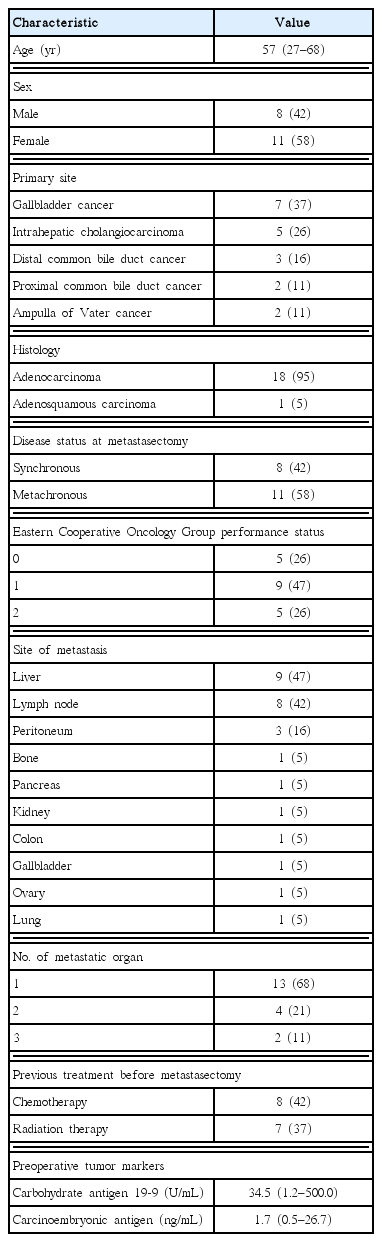
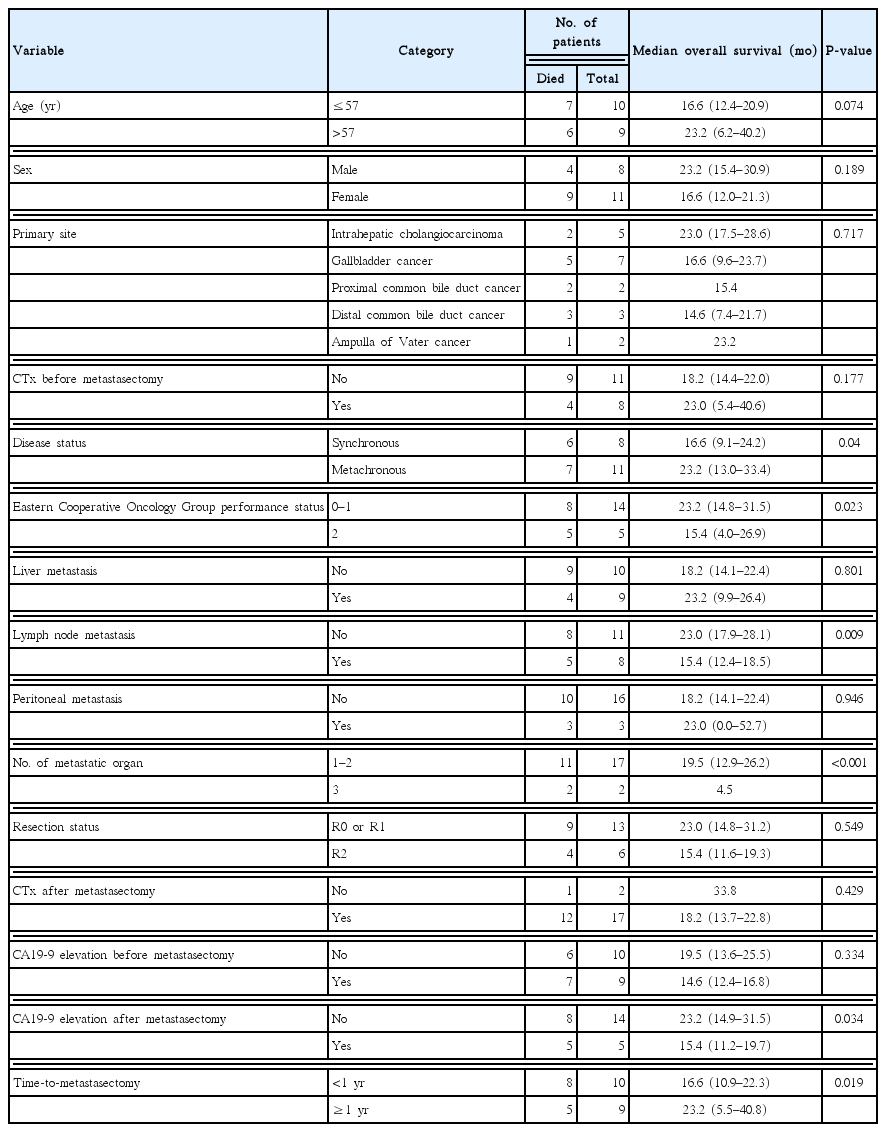
We found 19 eligible patients. Median age of patients was 57 years old (range, 27 to 68 years old), and 11 patients (58%) were female. Primary sites were gallbladder cancer (seven patients, 37%), intrahepatic cholangiocarcinoma (five patients, 26%), distal common bile duct cancer (three patients, 16%), proximal common bile duct cancer (two patients, 11%), and ampulla of Vater cancer (two patients, 11%). Eight patients (42%) had synchronous metastasis, while 11 (58%) had metachronous metastasis. The most common metastatic site was liver (nine patients, 47%), lymph node (nine patients, 47%), and peritoneum (three patients, 16%). Nine patients (47%) achieved R0 resection, while four (21%) and six (32%) patients had R1 and R2 resection, respectively. With a median follow-up period of 26.7 months, the estimated median overall survival (OS) was 18.2 months (95% confidence interval [CI], 13.6 to 22.9 months). Lower Eastern Cooperative Oncology Group performance status (P=0.023), metachronous metastasis (P=0.04), absence of lymph node metastasis (P=0.009), lower numbers of metastatic organs (P<0.001), normal postoperative carbohydrate antigen 19-9 level (P=0.034), and time from diagnosis to metastasectomy more than one year (P=0.019) were identified as prognostic factors for a longer OS after metastasectomy.
Conclusion
For recurrent or metastatic BTCs, metastasectomy can be a viable option for selected patients.
INTRODUCTION
Biliary tract carcinomas (BTCs), comprising intrahepatic cholangiocarcinoma (IHCCC), proximal and distal common bile duct cancer (pCBDC and dCBDC), gallbladder cancer (GBC), and ampulla of Vater cancer (AoVC), are relatively rare cancers [1]; however, high mortality and incidence rates are still evident in some countries, including central and eastern Europe, Japan, Chile, India, and Korea [2].
Complete surgical resection is the only chance for cure; however, a limited number of patients present with resectable disease. Unfortunately, even after surgical resection, recurrence is frequently reported. Prognosis for recurrent or metastatic BTC is still poor, with a median overall survival (OS) of around one year, even with the best treatment option [3].
Metastasectomy, resection of distant metastasis, has been increasingly adopted in some types of cancers, and it is associated with prolonged relapse free survival and even cure in prudently selected patients. Some examples of cancers in which metastasectomy is actively performed are colorectal cancer (hepatic metastasis and pulmonary metastasis) [4,5], soft tissue sarcoma (pulmonary metastasis) [6], and renal cell carcinoma (pulmonary metastasis) [7].
Efficacy or long term-result of metastasectomy for recurrent or metastatic BTC is not well established. Aggressive surgical attempts for advanced GBC have been reported several times [8–15]; however, most of these reports included patients with locally advanced GBC rather than metastatic GBC. Regarding metastasectomy in BTC, to the best of our knowledge, only a small number of cases involving a very limited number of patients have been reported [16–21]; therefore, the efficacy of the surgical resection of metastasis in BTC remains unclear.
Against this background, we retrospectively reviewed the outcomes of metastasectomy for recurrent or metastatic BTC in a single tertiary referral center hospital.
MATERIALS AND METHODS
1. Materials
The medical records of consecutive patients with BTCs (IHCCC, pCBDC, dCBDC, GBC, and AoVC) who were treated with surgery, chemotherapy, radiotherapy, or best supportive care at a tertiary referral hospital from 2003 to 2013 were reviewed retrospectively. In these patients, the selection criteria for analysis were as follows: histologically confirmed BTC; synchronous (M1 according to the American Joint Committee on Cancer 7th edition) or metachronous (recurrent) metastatic disease; and surgical resection of primary and metastatic sites. To be eligible for analysis, patients also had to have proper baseline and follow-up image work-up data before and after metastasectomy. Patients treated with metastasectomy for symptom palliation only were not included in analysis.
2. Data collection
Clinicopathological data, including age, sex, Eastern Cooperative Oncology Group performance status (ECOG PS), date of diagnosis, tumor histology, surgery, pathological stage, completeness of metastasectomy (R0, R1, or R2), metastatic site, prior treatment before metastasectomy, and laboratory data when metastasectomy was employed were collected. R0 is defined as no residual tumor, while R1 and R2 are defined as a microscopic residual tumor and macroscopic residual tumor, respectively. A comprehensive review of the preoperative baseline radiographic images and the follow-up radiographic images was performed. Preoperative images were usually performed within a month prior to metastasectomy, and postoperative disease status was evaluated by chest X-ray or chest computed tomography (CT) and CT of the abdomen and pelvis every 3 to 6 months. Tumor responses to those who received postoperative chemotherapy and/or radiotherapy were assessed every 2 to 3 months by CT. The type of subsequent treatment after metastasectomy and survival after metastasectomy were also reviewed. This study was approved by the institutional review board of Gil Medical Center (GBIRB2014-340).
3. Statistical analyses
Baseline patient characteristics and outcomes of metastasectomy were evaluated using descriptive statistics. The primary objectives of this study were OS from the date of metastasectomy. Descriptive statistics were used for reporting the baseline patient characteristics. Kaplan-Meier estimates were used in the analysis of time-to-event variables. Relapse-free survival (RFS) and OS were defined as the time from the date of metastasectomy to the date of recurrence and death from any cause, respectively. Time-to-metastasectomy (TTM) was defined as the time from the date of surgery for primary site to the date of the metastasectomy. Univariable analysis with log-rank test was performed for determination of the predictive and prognostic role of clinical factors (including age, sex, primary site, disease status, previous treatment before metastasectomy, resection status of metastasectomy, site of resection, laboratory values, etc.) associated with OS or RFS. Statistical analyses were performed using IBM SPSS ver. 19.0 (IBM Co., Armonk, NY, USA) and P<0.05 (two-sided) was considered statistically significant.
RESULTS
1. Patient characteristics
We found 19 eligible patients from 284 consecutive patients who were managed for BTCs from 2003 to 2013. The baseline characteristics of 19 patients meeting the selection criteria are shown in Table 1. Median age of patients was 57 years old (range, 27 to 68 years old), and 58% of patients were female. Primary sites were GBC (seven patients, 37%), IHCCC (five patients, 26%), dCBDC (three patients, 16%), pCBDC (two patients, 11%), and AoVC (two patients, 11%), in order. Most patients had adenocarcinoma (95%), eight (42%) had synchronous metastasis, and 11 (58%) had metachronous metastasis. Most patients had good ECOG PS before metastasectomy, as more than 70% had ECOG PS 0–1. The most common metastatic site was liver (nine patients, 47%), lymph node (nine patients, 47%), and peritoneum (in the form of limited peritoneal implantation without evidence of disseminated peritoneal seeding (three patients, 16%).
2. Outcomes of metastasectomy
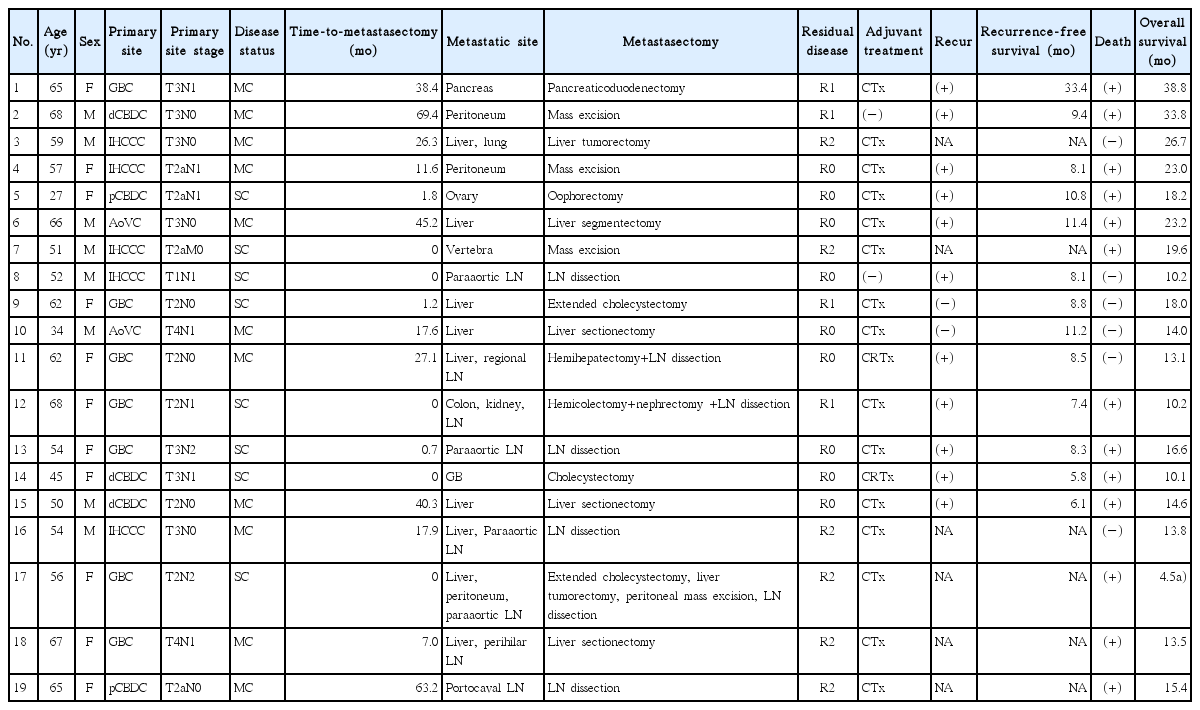
The clinical characteristics and outcome of metastasectomy are shown in Table 2. Nine patients (47%) achieved R0 resection, while four (21%) and six (32%) patients had R1 and R2 resection, respectively. Of six patients with R2 resection, four cases were due to bulky or severely adhesive lymph node metastasis and two cases were due to metastases in other organs (multiple small lung masses in one case and multiple bone metastases in another). There was no occurrence of metastasectomy-related death within one month after surgery. Seventeen patients received postoperative chemotherapy consisting of 5-fluorouracil or gemcitabine based chemotherapy with or without platinum. For the 13 patients with no grossly residual tumor (R0 or R1 resected), 10 patients experienced disease recurrence at the data cut-off, with a median RFS of 9.4 months (95% CI, 7.8 to 11.0 months). At the time of this analysis, 13 out of 19 patients had died. With a median follow-up period of 26.7 months, the estimated median OS was 18.2 months (95% CI, 13.6 to 22.9 months) (Fig. 1).
3. Prognostic factors and characteristics of patients with longer overall survival
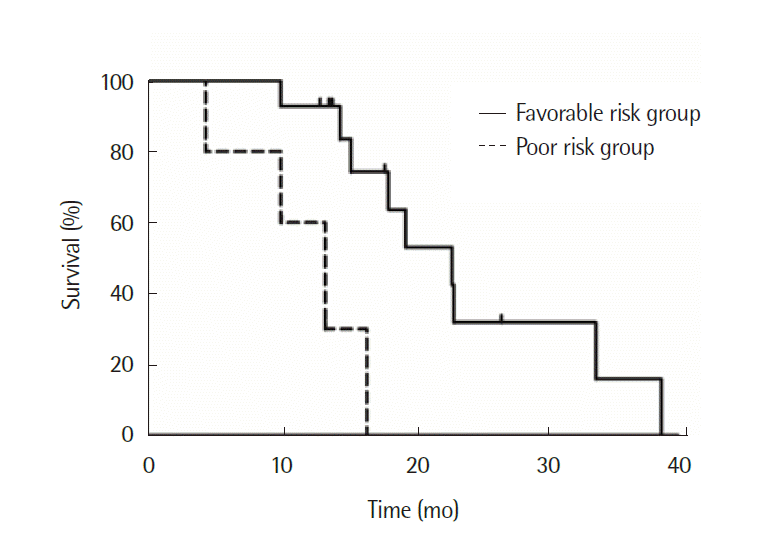
For patients who achieved R0 or R1 resection, predictive factors for RFS were explored using the log-rank test; however, no clinicopathologic factor was proved to be relevant to better PFS. For all patients who underwent metastasectomy, prognostic factors for OS were also explored. Lower ECOG PS (P=0.023), metachronous metastasis (P=0.04), absence of lymph node metastasis (P=0.009), lower numbers of metastatic organs (P<0.001), normal postoperative carbohydrate antigen 19-9 (CA 19-9) level (P=0.034), and TTM more than one year (P=0.019) were identified as prognostic factors for a longer OS after metastasectomy (Table 3). Primary tumor location did not show an association with OS (P=0.717). When categorizing patients according to favorable risk group (four or more prognostic factors) and poor risk group (three or less prognostic factors), OS of the favorable risk group was better with statistical significance, with a median OS of 23.0 months (95% CI, 16.1 to 30.0 months) vs. 13.5 months (95% CI, 8.3 to 18.6 months) (P=0.003) (Fig. 2).
DISCUSSION
In the current study, we found 19 patients with metastatic or recurrent BTC who underwent metastasectomy. Median OS was 18.2 months (95% CI, 13.6 to 22.9 months), and patients with lower ECOG PS, metachronous metastasis, absence of lymph node metastasis, lower numbers of metastatic organs, normal postoperative CA19-9 level, or TTM more than one year had longer OS after metastasectomy.
Previous reports of aggressive surgery in advanced GBC mostly included stage IVA and only a small fraction of stage IVB. It appears that aggressive surgery resulted in long-term survival in a very selected population, if curative resection is possible and number of metastases is limited. [11,12,14,15]. Some reports on metastasectomy for BTC include resection of adrenal metastasis from IHCCC [19], resection of solitary hepatic recurrence of IHCCC [20], and resection plus radiotherapy for two patients with locoregional failure of main hepatic duct carcinoma [21]. To date, data are insufficient to draw a conclusion regarding whether or not metastasectomy is helpful for patients with recurrent or metastatic BTC.
In a real world practice, clinicians are often faced with a dilemma when patients with BTC present with single or limited number of metastases. Because high level evidence is lacking, recommendation of metastasectomy is not usual. On the other hand, referring to some previous reports on metastasectomy, some selected patients can survive for a lengthy time by the benefit of metastasectomy. For definitive confirmation of efficacy of metastasectomy, conduct of a prospective randomized controlled trial comparing metastasectomy with no metastasectomy is needed; however, rarity of disease and difficulties in patient recruitment have precluded such a trial from being conducted. In fact, even in colorectal cancer, in which metastasectomy is performed very widely, no randomized controlled trial comparing surgery with no surgery has been reported. Despite no prospective randomized trials, as evidence supporting metastasectomy from colorectal cancer is mounting [22], metastasectomy is now a standard of care when feasible, and indication for metastasectomy is expanding with the passage of time.
Recent data on hepatic metastasectomy from colorectal cancer metastasis in the United States and Canada have demonstrated 10-year disease-free survival of 15% to 25%, which is much better than the survival on even the best systemic chemotherapy during that period [22]. Likewise, pulmonary metastasectomy for soft tissue sarcoma achieved a 3- and 5-year OS rate of approximately 45% and 35%, respectively, which is better than that for patients who did not undergo resection [6,23]. Lessons from such solid malignancies have shed light on the value of surgical resection in metastatic disease from other malignancies, and metastasectomy strategy could be adopted for advanced BTC with caution.
Because there will be sparse chance for conduct of a randomized controlled trial on metastasectomy in BTC, clinicians can make a decision regarding whether or not to perform metastasectomy based on extrapolated criteria from previous reports. In general, metastasectomy is preferred in the following situations: possibility of complete resection, small number of metastatic lesions (single metastasis or oligometastases rather than multiple metastases), no unresectable metastasis in other organs, and good general condition of patient.
In a previous report on aggressive surgery for BTC, some clinicopathologic features were described as good prognostic factors: low preoperative carcinoembryonic antigen [11], adjuvant chemotherapy [11], curative resection [11,15], isolated liver metastasis [14], absence of lymph node metastasis [15], absence of vascular resection [15], or absence of hepatoduodenal ligament invasion [15]. In the current study, patients with good ECOG PS, metachronous metastasis, absence of lymph node metastasis, lower numbers of metastatic organs, normal postoperative CA19-9 level, or TTM more than one year were depicted as good prognostic factors for longer OS after metastasectomy. Patients in the favorable risk group who had four or more good prognostic factors achieved significantly prolonged OS after metastasectomy. We could not perform multivariable analysis due to a small number of included patients; however, we believe that our results can be helpful in suggestion of some future criteria for metastasectomy in BTC.
There have been some suggestions that BTC is not a single entity of disease but a heterogeneous group of diseases with different prognoses [24] and biology [25] according to primary site. Therefore, metastasectomy can have an important role in some diseases while not in others. In addition, after metastasectomy, when visible masses are completely resected, the role of postoperative chemotherapy and/or radiotherapy is not known. In our study, primary site and post-metastasectomy treatment did not affect OS; however, we could not draw a firm conclusion due to the limitation of this study. In the future, if metastasectomy becomes a treatment option for at least some subsets of patients with advanced BTC, outcome differences among primary sites and the role of postoperative treatment should be verified.
There are several limitations in this study. The current study includes only a small number of patients, consisting of a heterogeneous group. In addition, this is a retrospective cohort study that included patients over many years, so that there could be biases inherent to this type of study. These shortcomings need to be rectified in future studies.
In conclusion, for recurrent or metastatic BTC, metastasectomy can be a viable option for selected patients, particularly those having good performance status and metachronous disease without recurrence of lymph node metastasis more than one year after surgery.