Endobronchial Neurilemmoma Mimicking a Bronchial Polyp
Article information
Abstract
Neurilemmomas are relatively uncommon, slowly growing tumors which originate from Schwann cells. Intrathoracic neurilemmomas often occur in the chest wall and posterior mediastinum, but endobronchial neurilemmomas are exceedingly rare. These tumors in trachea or bronchus are usually detected by radiologic examinations, mostly computed tomography scan of chest. An 88-year-old man was admitted for management of pneumonia in left lower lobe and parapneumonic effusion. On bronchoscopic examination, there was a small polypoid nodule less than 1 cm in diameter mimicking an endobronchial inflammatory polyp at the bifurcation of the right anterior segmental bronchus and lateral segmental bronchus and under auto-fluorescence imaging, the nodule showed reddish brown area with defined margin. The bronchoscopic biopsy revealed that the bronchial nodule was endobronchial neurilemmoma. This is an interesting case of endobronchial neurilemmoma mimicking a bronchial polyp that is detected incidentally via bronchoscopy.
INTRODUCTION
Neurilemmomas has been known as benign and slowly growing tumors which originate from Schwann cells that arise in any nerve such as cranial nerves, spinal roots, or peripheral nerves [1]. Intrathoracic neurilemmomas often occur in the chest wall and posterior mediastinum. Over the last several decades, endobronchial neurilemmoma are extremely rare with only few case reports in the literature, so the exact frequency is unknown [2]. In addition, it is difficult to define the typical and common finding of endobronchial neurilemmoma on bronchoscopic or radiologic examination. The standard treatment of pulmonary neurilemmoma has been surgical resection. However, bronchoscopic tumor resection has been reported to be a new modality of effective treatments in some cases [3], although it is controversial to classify pulmonary neurilemmoma according to its site and extension for the selection of treatment choice between operation and bronchoscopic removal [4]. In this report, we present our experience on the endobronchial neurilemmoma mimicking a bronchial polyp that is incidentally detected via bronchoscopy in an 88-year-old man, which may shed new light on the previously known bronchoscopic finding of neurilemmomas.
CASE REPORT
An 88-year old man admitted to Chonbuk National University Hospital because of cough and productive sputum of 2 weeks duration. He also complained of mild dyspnea. He was a farmer in rural area and a current smoker with a 60-pack-year history. He had history of testicular diffuse large B cell lymphoma stage II and received 6 cycle of chemotherapy including rituximab 3 years ago and had received ongoing hormonal therapy due to prostate cancer diagnosed 1 year ago.
Vital sign was within normal range and the breathing sound was decreased at the left lower lung field on physical examination. Laboratory tests revealed high sensitive C-reactive protein (49.5 mg/L) and others were unremarkable. Chest posterioanterior image showed patched consolidation in the left lower lung field with blunting of the left lateral costophrenic angle (Fig. 1A) and left lateral decubitus view revealed the left pleural fluid shifting (Fig. 1B). A computed tomography (CT) scan of chest demonstrated ground grass opacity in the left lower lobe with fluid collection in the left pleural space. And there was no endobronchial lesion. An initial impression was community-acquired pneumonia in left lower lobe with parapneumonic effusion. Fiber-optic bronchoscopy revealed a small bronchial polypoid nodule (less than 1 cm in size) with smooth surface at the bifurcation of the right anterior segmental bronchus and lateral segmental bronchus, suspected to be an endobronchial inflammatory polyp (Fig. 2A). Under auto-fluorescence imaging (AFI), the nodule showed reddish brown area with defined margin on normal mucosa appearing green color (Fig. 2B). Bronchoscopic biopsy of the bronchial polyp was performed. Interestingly, histopathologic examination of the biopsy material revealed spindle cells with a palisading pattern and positive result for S100 immunoperoxidase stain while negative for α-smooth muscle actin stain which was distinguishable from leiomyoma or meningioma (Fig. 3). On the basis of these findings, we diagnosed the bronchial polypoild nodule as endobronchial neurilemmoma. After the removal of endobronchial neurilemmoma with proper antibiotic therapy for pneumonia, the patient was discharged.

(A) Chest posterioanterior image reveals patched consolidation in the left lower lung field with blunting of the left lateral costophrenic angle. (B) Left lateral decubitus view shows the left pleural fluids shifting.
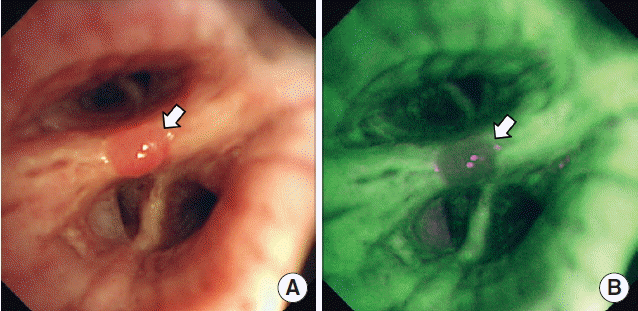
(A) White light videobronchoscopy revealed a small bronchial polypoid nodule (white arrow) with smooth surface at the bifurcation of the right anterior segmental bronchus and lateral segmental bronchus. (B) Under auto-fluorescence imaging, the nodule showed reddish brown area with defined margin on normal mucosa appearing green color (white arrow).
DISCUSSION
Most of lung tumors are malignant and less than 5% of them are benign such as adenoma, papilloma, and hamartoma [5]. Although being exceedingly rare, pulmonary neurilemmomas can occur in all respiratory tracts including trachea, bronchus, bronchioles, and alveoli as one of benign lung tumors [2,6]. However, only scattered cases of endobronchial neurilemmomas have been reported in Korea [7].
The clinical presentation of endobronchial tumors varies and depends on the size, location, and degree of bronchial obstruction. Therefore, neurilemmomas in bronchial tree show a variety of respiratory symptoms including cough, dyspnea, hemoptysis, and wheezing and often have no symptoms [3]. They usually have a long natural history, causing symptoms only after they have attained a large size [8]. Because symptoms are non-specific, endobronchial neurilemmoma cannot be diagnosed on the basis of clinical presentation. Our patient had cough, sputum, and dyspnea, but these symptoms were considered to be caused by pneumonia in the left lower lobe and left pleural effusion. Endobronchial neurilemmoma was too small to induce bronchial obstruction or respiratory symptoms.
The contrast enhanced CT of chest is useful in assessing lung parenchyma and inside the lumen as well as outside the respiratory tract. On CT scan of chest, most cases of endobronchial neurilemmomas show endobronchial mass, pulmonary nodule, atelectasis, and pneumonic infiltration [3,7]. The median diameter of the tumors was 2.5 cm (range, 2 to 3 cm) [7]. Many cases of endobronchial neurilemmomas have been detected by CT scan and subsequently diagnosed via bronchoscopy [7]. However, the endobronchial neurilemmoma in our case was incidentally detected via bronchoscopy during the evaluation of pneumonia and pleural effusion, as the size of bronchial tumor was so small.
The bronchoscopic findings of endobronchial neurilemmoma are various and the characteristics are difficult to be defined. On bronchoscopic examination in previous reports, endobronchial neurilemmomas had usually larger size than our case and appeared a hypervascular mass [7] and rare cases presenting with polypoid mass were broad-based tumors [5]. In contrast to these previous cases, endobronchial neurilemmoma in our patient was presented with a small polypoid nodule less than 1 cm in diameter, which was suspected to be an endobronchial inflammatory polyp. Endobronchial inflammatory polyps are usually related to chronic inflammatory processes in the adult and presented with solitary benign endobronchial lesions with stromal configurations consisting of well-formed fibrous connective tissue with or without inflammatory cell infiltration [9,10]. As the endobronchial polypoid nodule in our case was the smallest one reported ever and endobronchial neurilemmomas have been extremely rare, we could not consider neurilemmoma with suggesting an inflammatory polyp.
Our report has provided the AFI finding of endobronchial neurilemmoma. AFI and narrow band imaging have more potential for detection of precancerous lesions over conventional white light bronchoscopy and are now widely used as a scientific bronchoscopic tool for evaluation of endobronchially visible tumors [11]. AFI can successfully ascertain intraepithelial changes and is mainly constructed to detect weak autofluorescent signal from pathologically altered bronchial mucosa [12]. Under AFI, pathologic areas appear reddish brown or magenta color, while normal areas appearing green. Conditions such as metaplasia, hyperplasia, and inflammation of the bronchial tissue may produce false positive results with AFI [13]. There is no available information regarding the use of AFI for evaluation of neurilemmoma. The endobronchial neurilemmoma in our case showed the pathologically altered mucosa under AFI. As far as we know, this report is the first to show AFI finding of endobronchial neurilemmoma.
In our case, the endobronchial neurilemmoma was incidentally diagnosed via bronchoscopy during the evaluation and treatment of pneumonia. The tumor was located in the right lower lobe bronchus while pneumonic consolidation being in the left lower lobe, suggesting that the tumor was not related with pneumonia. On bronchoscopic examination, the endobronchial neurilemmoma in our case presented as a polypoid nodule with smaller size than these tumors previously reported, suspected to be an endobronchial inflammatory polyp. In addition, the endobronchial neurilemmoma showed the pathologically altered mucosa under AFI, which was the first report showing AFI finding of this tumor. Even though there are still many debates on the clinical significance and characteristic findings on radiological and bronchoscopic examinations, endobronchial neurilemmomas may be considered as one of differential diagnoses in a patient with endobronchial nodule.
ACKNOWLEDGMENTS
This paper was supported by Fund of Biomedical Research Institute, Chonbuk National University Hospital.
