A Case of Allergic Bronchopulmonary Aspergillosis Forming Broncholith Misdiagnosed as an Uncontrolled Asthma with Broncholithiasis
Article information
Abstract
A 55-year-old woman was referred to the division of pulmonology at Severance Hospital for the investigation of uncontrolled asthma with recurrent cough at night, blood-tinged sputum, malaise, and wheezing since 3 months. Chest computed tomography revealed bronchiectasis and broncholithiasis in the lateral segmental bronchus of the right middle lobe and the anterobasal segmental bronchus of the right lower lobe. Bronchoscopic broncholith removal was performed because of recurrent blood-tinged sputum and the outflow of purulent exudate behind the broncholith in the lateral segmental bronchus of the right middle lobe. The extracted material presenting amorphous eosinophilic necrotic materials with calcification was compatible with broncholithiasis. Following decalcification, histopathology revealed degenerated septate fungal hyphae and spores that were morphologically consistent with Aspergillus spp. A final diagnosis was allergic bronchopulmonary aspergillosis (ABPA) forming broncholith. The results from this case suggest that the early recognition of ABPA should be considered in patients with uncontrolled asthma accompanied by broncholithiasis.
INTRODUCTION
Uncontrolled asthma showing a poor response to treatment should be considered for differential diagnoses such as chronic obstructive pulmonary disease, bronchiolitis, gastroesophageal reflux disease, recurrent aspiration, central airway obstruction, side effects of drugs (e.g., angiotensin-converting enzyme inhibitor-induced cough), pulmonary embolism, and allergic bronchopulmonary aspergillosis (ABPA) [1]. Especially, ABPA is estimated to affect 1% to 2% of the asthmatic population [2]. In patients with steroid-dependent asthma, its prevalence is believed to be 7% to 14% [3]. Early recognizing ABPA is mainly based on clinical suspicion, which is related with improvement of patient symptoms and prevention of sustained lung damage in ABPA [4]. However, symptomatic broncholithiasis can cause confusion or delay in diagnosing ABPA because diagnostic criteria did not contain the existence of broncholithiasis. In addition ABPA with broncholithiasis is an extremely rare condition. Herein we report a case of a 55-year-old woman with uncontrolled asthma with broncholithiasis who was finally diagnosed with ABPA forming broncholith.
CASE REPORT
A 55-year-old woman was referred to the division of pulmonology at Severance Hospital for the investigation of uncontrolled asthma with recurrent cough at night, blood-tinged sputum, and wheezing since 3 months. Right middle lobe microcalcification was confirmed on initial chest radiographs (Fig. 1A). According to the results of pulmonary function tests, asthma was considered a reasonable diagnosis because of the obstructive pattern and positive bronchodilator response (Fig. 1B). Maxillary sinusitis was identified on Water’s view for the paranasal sinuses (Fig. 2), and moderate eosinophilia (14%) was confirmed in induced sputum analysis. Sputum acid-fast bacilli smear, culture and polymerase chain reaction (PCR) for tuberculosis and non-tuberculosis mycobacterium were negative. Although the patient was treated for asthma, sinusitis, and bronchitis, her symptoms did not improve. She just responded to intermittent systemic corticosteroid therapy for uncontrolled asthma.

(A) Chest radiograph showing calcification in the right middle lobe. (B) Pulmonary function test revealing mild obstructive pulmonary insufficiency with positive bronchodilator response. FVC, forced vital capacity; FEV1, forced expiratory volume in one second; FEF, forced expiratory flow; PEF, peak expiratory flow; FET, forced expiratory time; FIF, forced inspiratory flow.

Computed tomography of the paranasal sinuses showing bilateral chronic maxillary sinusitis and nasal septal deviation to the right.
Therefore, for the differential diagnosis of uncontrolled asthma, chest computed tomography (CT) was performed, which revealed bronchiectasis and broncholithiasis in the lateral segmental bronchus of the right middle lobe and the anterobasal segmental bronchus of the right lower lobe (Fig. 3). Bronchoscopic broncholith removal was scheduled because of recurrent blood-tinged sputum and the outflow of purulent exudate behind the broncholith in the lateral segment bronchus of the right middle lobe (Fig. 4A, B). Using lithotomy forceps with a basket, the approximately 1-cm-sized broncholith was completely extracted (Fig. 4C) and presented amorphous eosinophilic necrotic materials with calcification, compatible with pathologic finding of broncholithiasis. Following decalcification, histopathological examination of the specimen showed degenerating septate fungal hyphae and spores that were morphologically consistent with Aspergillus spp. (Fig. 4D). Thus we performed additional tests for the confirmation of ABPA. Peripheral eosinophilia (11.4%, 0.66×103/μL), a total immunoglobulin E (IgE) level of 126 kU/L (normal, <100 kU/L and >417 kU/L in ABPA), and positivity for serum antibodies specific to Aspergillus fumigatus, namely m3 IgE (2.92 kU/L; normal, <0.35 kU/L) and Gm3 immunoglobulin G (IgG, 22.8 mg/L; normal, <39 mg/L), were observed. The findings of asthma, central bronchiectasis, elevated serum levels of IgE antibodies to Aspergillus fumigatus, and the absence of infiltration on chest radiographs confirmed a diagnosis of ABPA. We believed that the intermittent systemic oral corticosteroids may have decreased the total IgE levels and the serum levels of IgG antibodies to Aspergillus fumigatus. She discharged after one weeks of conservative care and broncholith removal.

Chest computed tomography showing bronchiectasis in the right middle lobe, lingular segment of the left upper lobe, and both the lower lobes, with mucus plug formation. (A) Broncholith in the lateral segmental bronchus of the right middle lobe and anterobasal segmental bronchus of the right lower lobe. (B) Centrilobular ground-glass opacity and nodules in the right middle lobe.
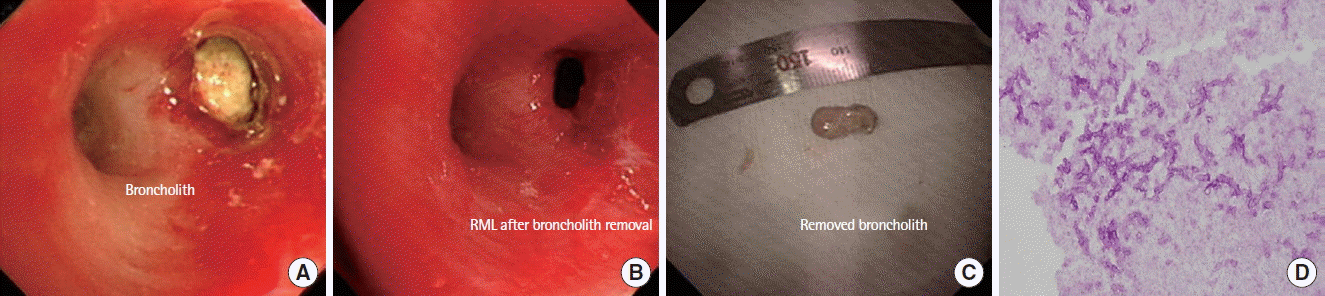
(A) Bronchoscopy showing a brocholith in the medial segment of the RML. (B) Opening of the medial segment of the RML after brochoscopic removal of the broncholith. (C) A broncholith measuring approximately 1.2 cm in size. (D) Periodic acid–Schiff staining after decalcification of the removed broncholith showing degenerated septate fungal hyphae and spores (× 400). RML, right middle lobe.
The patient showed symptomatic and radiographic improvement after broncholith removal (Fig. 5). And the administration of oral corticosteroid, itraconazole for 6 months (initial prednisolone 30 mg and itraconazole 400 mg) was taperd down over the next 6 months. At the moment, she is under observation with inhaled corticosteroid, antihistamine, and leukotriene receptor antagonist.
DISCUSSION
We reported a case involving a 55-year-old woman with symptoms of uncontrolled asthma with broncholithiasis who was finally diagnosed with ABPA forming broncholith. This is an interesting and rare case of uncontrolled asthma accompanied by broncholithiasis, which results in delay diagnosis of ABPA only after pathologic diagnosis of fungal hyphae through removal of broncholith.
In patients with uncontrolled asthma in particular, ABPA should be considered as a complicated disease or differential diagnosis. Generally, ABPA is clinically characterized by poorly controlled asthma, recurrent pulmonary infiltration, and bronchiectasis. The Rosenberg–Patterson criteria used as the primary diagnosis for ABPA includes clinical, radiological, and serological manifestations [2,5,6]. This criteria are composed of eight major criteria such as (1) a history of asthma, (2) central bronchiectasis, (3) a positive aspergillus skin test, (4) peripheral eosinophilia, (5) precipitin antibodies in the serum, (6) a serum IgE level of >1,000 mg/mL, and (7) Aspergillus fumigate-specific IgG and IgE antibodies in the serum, (8) lung infiltration on chest X-ray or CT scan and three minor criteria, namely the presence of Aspergillus hyphae in sputum, expectoration of black mucus plugs, and a delayed skin reaction to the Aspergillus antigen that may not be evident during remission or in the fibrotic stage [7]. ABPA can be divided into five stages, with each stage showing a different pattern of presentation as follows: stage I, acute disease; stage II, remission; stage III, exacerbation; stage IV, corticosteroid-dependent asthma; and stage V, fibrotic end stage disease [8]. However, there is no individual test to diagnose ABPA. Therefore clinical suspicion and early detection can reduce the risk of progression to fibrotic disease. Although we did not recognize ABGA until decalcified broncholith revealed aspergillosis, our patient fulfilled the diagnostic criteria for ABPA by exhibiting asthma, bronchiectasis, peripheral eosinophilia, and positive precipitating serum antibodies to Aspergillus fumigatus. However, in our case, the focal central bronchiectasis combined with broncholithiasis was not typical central bronchiectasis in other ABPA cases.
Broncholithiasis is defined as the presence of calcified or ossified material within the lumen of the bronchus. Although a broncholith is generally formed from an adjacent calcified lymph node and is usually associated with necrotizing granulomatous lymphadenitis or tuberculosis [9], broncholithiasis secondary to aspergillosis is rare. In our case, the results of tuberculosis PCR in sputum and bronchial washing were all negative. Apart from the present case, only one case of ABPA associated with a broncholith has been reported in Korea [10]. However, ABPA diagnosis of this case was obscure because asthma, which is the major criteria for ABPA, was not fulfilled. Therefore, ABPA forming broncholith in uncontrolled asthma with broncholithiasis must be unique case.
In conclusion, ABPA with broncholithiasis is an extremely rare condition. To the best of our knowledge, this is the second reported case of ABPA forming broncholithiasis in Korea and will contribute to the literature because its unusual presentation will be helpful for early recognition of ABPA. We recommend that clinicians should consider ABPA forming broncholith, particularly in patients with uncontrolled asthma and broncholithiasis.
