Consideration of Discrepancy between Needle-Washout Thyroglobulin and Serum Thyroglobulin of Recurrent Papillary Thyroid Cancer
Article information
Abstract
Although the prognosis of papillary thyroid cancer (PTC) is extremely good, locoregional recurrences after initial treatment occur. Thyroglobulin (Tg) is a reliable tumor marker to detect recurrence or persistence of PTC. However, occasionally serum Tg may miss the detection of a recurrence. We report a 54-year-old female presented with hoarseness due to cervical recurrence without concomitant elevation of serum Tg and anti-Tg antibody, in contrast to extremely increased needle-washout Tg, who had undergone a total thyroidectomy and radioiodine ablation as initial therapies for PTC. Several factors causing such discrepancy between needlewashout Tg and serum Tg can be suggested including site of recurrence, volume of tumor, interference by some kind of plasma antibodies other than anti-Tg antibody, and any conformational defect of Tg protein. Among them, the most convincing explanation is that any conformational defect of Tg may lead to impaired secretion of Tg to blood. We suggest that more studies are needed to find the cause for potential mechanisms involved in PTC recurrences without increased serum Tg.
INTRODUCTION
Although the vast majority of patients with papillary thyroid cancer (PTC) have an excellent prognosis, disease recurrences do occur. During follow-up after initial surgery and/or radioiodine therapy, serum thyroglobulin (Tg) measurement, neck ultrasound, and/or diagnostic whole-body radioiodine scan are recommended depending on the risk for recurrence in a patient with PTC [1,2]. Particularly, serum Tg is the most sensitive biomarker of recurrence for well-differentiated thyroid cancer. Since Tg is synthesized only by thyroid follicular cells, measurements of serum Tg provide important information about the presence of residual, recurrent, or metastatic disease in patients with differentiated thyroid cancer [3]. However, occasionally serum Tg may miss the detection of a recurrence. Here, we report a case of cervical recurrence without a concomitant increase in serum Tg and anti-Tg antibody in a patient with PTC who had undergone a total thyroidectomy and radioiodine ablation as initial therapies.
CASE REPORT
A 54-year-old female with suspicious malignant nodule in thyroid was referred to Soonchunhyang University Hospital. The initial serum thyroid-stimulating hormone (TSH) level was 0.92 mU/ L (reference range, 0.40 to 5.00 mU/L), and the preoperative levels of serum Tg (IRMA kit; Cisbio Bioassay, Codolet, France; functional sensitivity, 0.7 μg/L) and anti-Tg antibody (RIA kit; RSR Ltd., Cardiff, UK) were 20.82 μg/L (reference range, 0 to 35 μg/L) and negative (reference range, 0 to 60 μg/L), respectively. Thyroid ultrasound revealed a calcified nodule in left lobe. An ultrasound-guided fine needle aspiration (FNA) biopsy results showed suspicious for PTC. She underwent a total thyroidectomy and central neck dissection with preserving the recurrent laryngeal nerve for thyroid cancer in September 2009. In the thyroidectomy specimen, a 1.6- cm tumor in the left lobe was noted. Microscopic findings showed proliferation of papillary structures or follicles lined by cuboidal tumor cells with enlarged ground glass nuclei, irregular nuclear outline, nuclear grooves, and occasional intranuclear inclusion on the sclerotic stroma, consistent with typical type of papillary carcinoma. It spread to the perithyroidal soft tissue (Fig. 1A). Dissected lymph nodes showed reactive hyperplasia without tumor metastasis. In December 2009, she received radioiodine ablation with 150 mCi of 131I. A post-therapy whole-body scan showed only focal uptake in the neck (Fig. 2). Stimulated serum Tg level obtained after thyroxine withdrawal was 9.56 μg/L and serum TSH level was 64.81 mU/L. She was followed by clinical examination, assessment of serum Tg levels and anti-Tg antibody, and ultrasound of the neck every 6 to 12 months without evidence of disease. TSH suppression therapy by levothyroxine was maintained to achieve a serum TSH less than 0.10 mU/L (Table 1).
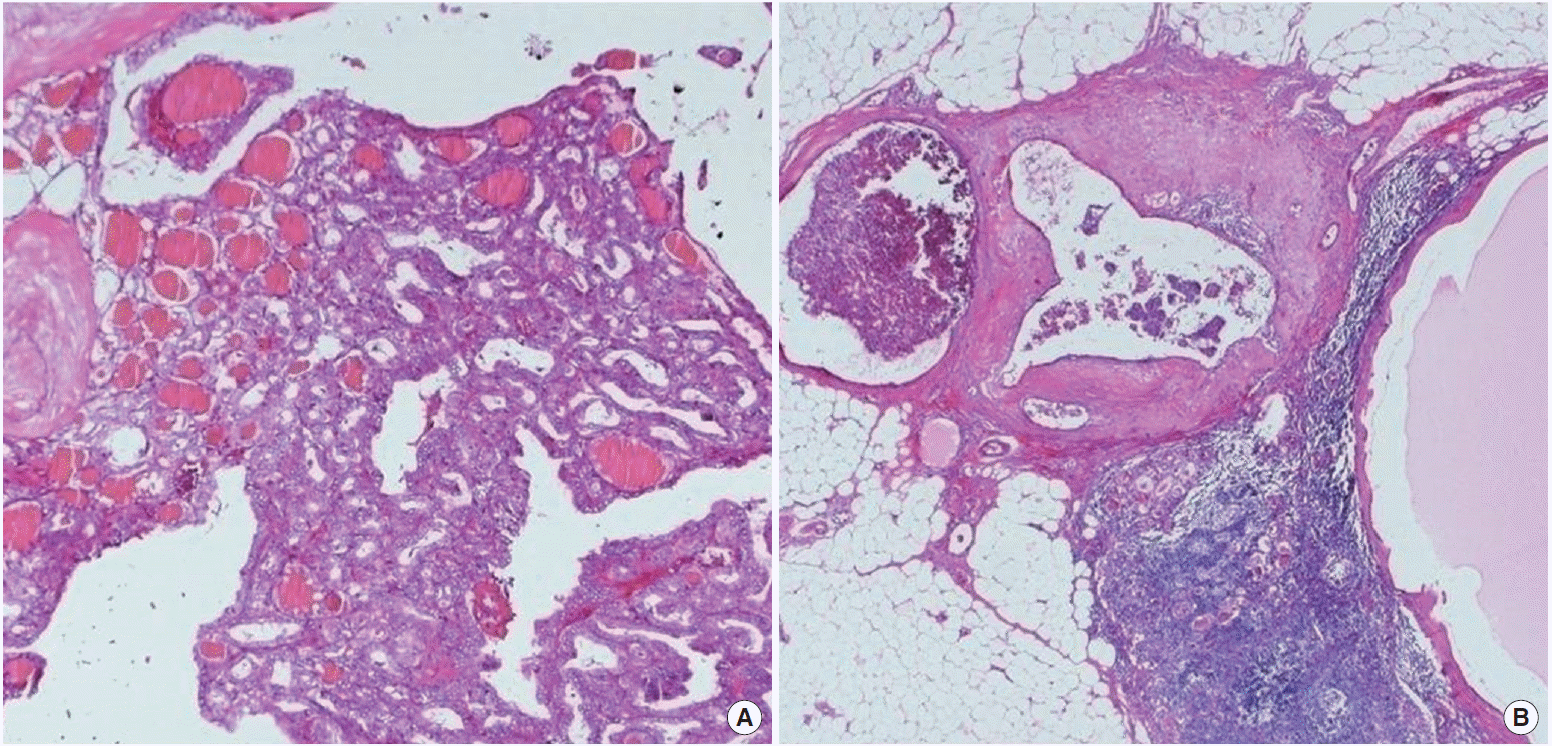
Microscopic findings of primary tumor and metastatic lesions. It shows follicular to papillary proliferation of tumor cells on the sclerotic background. (A) Their nuclei have enlarged characteristic ground glass nuclei and nuclear grooves (H&E, × 100). (B) The recurrent tumor nodules of the neck are lymph nodes involved by metastatic papillary carcinoma of the thyroid (H&E, × 40).
At 3 years after surgery, she complained of hoarseness for the past 6 weeks. Laryngoscopic examination showed left vocal cord palsy. Thyroid ultrasound revealed two small lymph nodes at right level IV and a 1.2-cm sized mass at left level VI suspicious for lymph node metastasis. The ultrasound-guided FNA was performed, and the needle-washout fluid was analyzed both cytological examination and Tg assay. The cytology specimen showed PTC recurrence. The level of needle-washout Tg was 3,825.3 μg/L in right level IV and 3,355.7 μg/L in left level VI. Serum TSH level was 0.02 mU/L, Tg was 0.09 μg/L, and anti-Tg antibody was negative at that time. Although the immunoassay used is protected against potential interferences like human anti-mouse antibodies by adding a protection in the tracer, it cannot be guaranteed that there will never be detection of ‘false positive’ or ‘false negative’ due to the presence of interferences like heterophile antibodies (HAB) in the patient samples. So, to determine whether HAB to Tg were present in our patient’s serum, we performed test by two methods. First, precipitation method using serum of the patient and four euthyroid controls was performed. The amount of 100 μL of each serum was incubated at 37°C for 1hour with 500 μL of the 125I-labelled Tg analog. The 1 mL of 20% polyethylene glycol (PEG) was added. Then, that was centrifuged at 3,000 rpm for 30 minutes. Radioactivity of the precipitate were measured using gamma counter. When the radioactivity of the precipitate was expressed as a percentage of total radioactivity, there were no significant differences between the patient and controls. Second, 50 μL of each serum and 1 mL of 125I-labelled Tg analog were mixed in anti-Tg antibody coated tube and shaken for 2 hours. The 1 mL of 30% PEG was added, then, centrifuged and measured radioactivity of each tube. There were no significant differences of radioactivity between the patient and controls. Selective neck dissection was performed for right lateral and left central neck. Pathology specimens were confirmed PTC at right level IV and left level VI, and histologic features are same as the initial mass (Fig. 1B). After surgery, she immediately recovered from hoarseness and vocal cords are fully adducted on laryngoscopic examination. Three months later, a dose of 150 mCi of 131I was administered and post-therapy whole-body scan demonstrated no abnormal increased uptake. Stimulated serum Tg level obtained after thyroxine withdrawal was 0.63 μg/L and serum TSH level was 25.75 mU/L. She is receiving TSH suppression therapy and followed without evidence of disease until now.
DISCUSSION
According to recent Korean [1] and American [2] management guideline for patients with thyroid cancer, measurements of serum Tg, anti-Tg antibody and TSH at 6- to 12-month intervals and neck ultrasound 6 to 12 months postoperatively and then periodically depending on the patient’s risk for recurrent disease are recommended. Tg is a highly sensitive and specific marker for detection of recurrent or residual disease after initial therapy [3]. After total thyroidectomy and radioiodine ablation, serum Tg levels would be expected to be very low (<1 to 2 μg/L), either on TSH suppression therapy or after thyroid hormone withdrawal, if the patient is cured [4]. As a single investigation, serum Tg measurement is more sensitive than radioiodine uptake scanning [5-9]. Previous studies suggested that patients with low levels of serum Tg during suppression therapy may have a high probability of being free of disease [10,11].
The present case represented PTC recurrences despite the absence of detectable serum Tg and the negativity of anti-Tg antibody contradictory to high needle-washout Tg level. There are several potential mechanisms that might be involved in PTC recurrence with such discrepancy between needle-washout Tg and serum Tg. First, tumor cells might be ‘poor secretor.’ It means tumor could have synthesized Tg but had some defects on secretion process. To date, 43 inactivating mutations have been reported in the human TG gene [12]. Some Tg mutants have defective intracellular transport and accumulate within the rough endoplasmic reticulum, so cannot be released into the follicle lumen [12-16]. We performed immunostaining for Tg from primary mass and recurrent lymph nodes specimens. Immunohistochemical stains demonstrated strong positive staining for Tg in the tumor cells and colloid of the both initial mass and metastatic nodule (Fig. 3). So it is less likely that tumor cells had some defect of intracellular transport of Tg to colloid. Instead, Tg protein may had any conformational change leading to failure of secretion from colloid to blood. It also may be the reason of negative serum Tg versus positive needle-washout Tg. Further pathologic examination by electron microscope may be needed. Second, the site of recurrence may influence serum Tg level. Patients with distant metastases to lung or bone have higher Tg levels than those with lymph node metastases [11]. Our patient had metastases only to cervical lymph nodes and no distant metastases. However, this fact may explain the result of negative serum Tg, but not that of high needle-washout Tg level. Third, the volume of metastatic tumor may be too small to increase serum Tg level enough [17,18]. Bachelot et al. [17] measured Tg level after thyroid hormone withdrawal and the tumor mass in thyroid cancer patients who underwent surgery with the use of an intraoperative probe for lymph node metastases with 131I uptake. Patients were classified into one of three groups according to the Tg level: undetectable (n=18); 1 to 10 ng/mL (n=21); and greater than 10 ng/mL (n=33). They concluded that total surface or total volume is the characteristic that best summarizes the influence of the disease on the serum Tg level. In undetectable Tg group, median total surface was 9.5 mm2 and mean total volume was 9.2 mm3. The estimated total surface and total volume of metastatic tumor in this case were 272 mm2 and 1,888 mm3. Therefore, the serum Tg levels of present case were oddly low considering tumor surface and volume. When we assume that there is any conformational defect on Tg leading secretory failure, it can be explained that serum Tg level is low despite of the relative large volume of tumor producing enough Tg. Fourth, interference by HAB may lead to false decreases and increases in Tg concentrations [19]. HAB interference is relatively prevalent (range, 1.5% to 3%) in a commonly used automated Tg assay, so HAB interference should be suspected if Tg results do not fit the clinical picture [19]. HAB did not significantly interfere with Tg measurement in FNA washout fluid, probably because of the very high Tg concentrations in the needle-washout fluids [20]. However, we could not identify HAB interference in patient’s serum.

Microscopic findings of primary tumor and metastatic lesions. It shows follicular to papillary proliferation of tumor cells on the sclerotic background. (A) Their nuclei have enlarged characteristic ground glass nuclei and nuclear grooves (H&E, × 100). (B) The recurrent tumor nodules of the neck are lymph nodes involved by metastatic papillary carcinoma of the thyroid (H&E, × 40).
The present case has some limitations. Most of all, serum Tg level after thyroid hormone withdrawal were not measured. However, it is usually adopted measuring of serum Tg on thyroxine because of its high sensitivity and the inconvenience of withdrawal of thyroid hormone. Furthermore, patients with a TSH-suppressed serum Tg concentration <0.1 μg/L were unlikely to have an stimulated Tg above 2.0 μg/L [21].
In summary, we report an unusual case of PTC recurrence without a concomitant increase in serum Tg and anti-Tg antibody, in contrast to extremely increased needle-washout Tg, in a patient who had undergone a total thyroidectomy and radioiodine ablation as initial therapies. It is important that PTC patients need to be followed by clinical examination and ultrasound of the neck as well as assessment of serum Tg levels and anti-Tg antibodies. We suggest that more studies are needed to find the cause for potential mechanisms involved in PTC recurrences without concomitant elevation of serum Tg levels in the absence anti-Tg antibody.

