Acute Pancreatitis Associated with Pegylated Interferon α-2b and Ribavirin Treatment of Chronic Hepatitis C
Article information
Abstract
Acute pancreatitis is an uncommon side effect of pegylated interferon (PEG-IFN) α-2b and ribavirin (RBV) combination therapy. In South Korea, There is a no report of acute pancreatitis associated PEG-IFN α-2b plus RBV combination therapy. Here, acute pancreatitis associated with PEG-IFN α-2b plus RBV treatment is described in two patients with chronic hepatitis C. We started on weekly subcutaneous injection of PEG-IFN α-2b plus daily RBV. During this therapy, acute pancreatitis occurred in these patients without other causes of acute pancreatitis. We thought that the cause of acute pancreatitis in these patients was PEG-IFN α-2b and RBV. We stopped the treatment of PEG-IFN α-2b and RBV, and patients were improved.
INTRODUCTION
According to World Health Organization, today 130 million people globally have chronic hepatitis C infection and 350,000 people die each year from hepatitis C-related liver disease [1]. In South Korea, 1% of total population is infected by hepatitis C virus (HCV). Approximately 20 to 30 percent of chronically infected individuals develop cirrhosis. The treatment of chronic hepatitis C (CHC) is very important for the prevention of cirrhosis and hepatocellular carcinoma.
Combination therapy of pegylated interferon (PEG-IFN) α-2b and ribavirin (RBV) was approved in 2001 in U.S. Food and Drug Administration and used in USA, and in Korea, this combination therapy started in 2004 and was listed on Korea National Health Insurance and this combination therapy is used in centers widely. During this therapy, some side effects can occur. Known side effects are neutropenia, gastrointestinal problems such as nausea, vomiting, depression, weight loss, insomnia, skin rash, etc. And acute pancreatitis can occur rarely. There is no report of acute pancreatitis associated PEG-IFN α-2b and RBV combination therapy in South Korea. This paper reports our experience of acute pancreatitis associated PEG-IFN α-2b and RBV combination therapy.
CASE REPORTS
1. Case 1
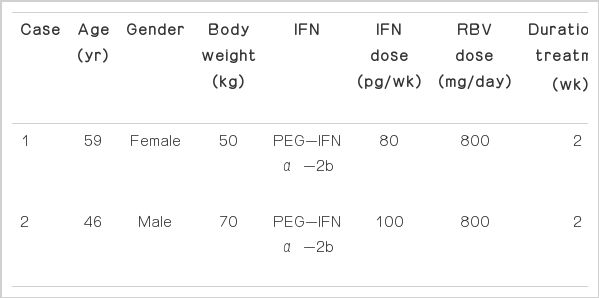
A 59-year-old female visited local medical clinic and her blood test showed positive finding of HCV antibody. She was referred to Yeosu Chonnam Hospital for further evaluation and management of positive finding of HCV antibody. She had gastric ulcer history but it was treated. She didn’t have other medical history and she was social drinker but did not drink recently. Laboratory finding showed HCV reverse transcription polymerase chain reaction (RT-PCR) test was positive. HCV RNA level was 1.27×107 IU/mL, genotype was 2a. We checked abdominal ultrasonography and abdominal computed tomography (CT), which showed mild fatty liver. We diagnosed chronic viral hepatitis C and started on weekly subcutaneous injection of 80 μg of PEG-IFN α-2b plus daily oral dose of 800 mg RBV. On 5th day after treatment, she had mild epigastric pain and nausea. We continued to treat but her symptoms worsened. She revisited hospital and was admitted. She had severe epigastric pain with radiating to her back, nausea, vomiting, and cold sweating. Laboratory finding showed the following: white blood cell (WBC) 5,600/mm3 (neutrophil 45.1%, lymphocyte 46.3%, monocyte 8.2%, and eosinophil 0.2%), aspartate aminotransferase (AST) 45 IU/L, alanine aminotransferase (ALT) 92 IU/L, alkaline phosphatase 84 IU/L, r-GTP (gamma-glutamyl transpeptidase) 63 IU/L, total-bilirubin 0.6 mg/dL, total-protein 7.1 g/dL, albumin 4.6 g/dL, blood urea nitrogen (BUN) 10 mg/dL, creatinine (Cr) 0.8 mg/dL, triglyceride 70 mg/dL, total calcium 9.8 mg/dL, amylase 239 U/L, and lipase 669 U/L. We rechecked abdominal ultrasonography. It showed mild fatty liver and no abnormality of pancreas, pancreatic and common bile duct, and gallbladder. She had no history of pancreatitis, denied alcohol use, and was not taking any medications. There was no reason to cause pancreatitis, except PEG-IFN α-2b and RBV therapy. We thought PEG-IFN α-2b and RBV was associated with pancreatitis. PEG-IFN α-2b and RBV therapy was stopped. She was fasted and treated with ulinastatin 100,000 IU/day. After treatment, amylase and lipase level fell to 121 IU/L and 287 IU/L. She began to eat again with soft meal and we restarted PEG-IFN α-2b and RBV. But next day, she complained epigastric pain again and the level of amylase and lipase rise to 245 IU/L and 557 IU/L. We decided to stop the PEG-IFN α-2b and RBV therapy. After discontinuation of treatment, her symptoms and lab findings improved. Pancreatitis did not recurred.
2. Case 2
A 46-year-old male referred to Yeosu Chonnam Hospital for treatment of chronic hepatitis C. His body weight was 70 kg. Local clinic test showed HCV RT-PCR test was positive and RNA level was 6.4×105 IU/mL. We checked the laboratory test which showed the following: WBC 4,660/mm3 (neutrophil 33.3%, lymphocyte 57.1%, monocyte 7.3%, and eosinophil 2.1%), AST 111 IU/L, ALT 162 IU/L, alkaline phosphatase 107 IU/L, r-GTP 95 IU/L, total-bilirubin 0.9 mg/dL, total-protein 8.4 g/dL, albumin 4.8 g/dL, BUN 12 mg/dL, Cr 0.8 mg/dL, triglyceride 141, total calcium 9.1 mg/dL, and HCV genotype was 2a. Abdominal CT showed chronic liver disease. He was admitted and we started on weekly subcutaneous injection of 100 μg of PEG-IFN α-2b plus daily oral dose of 800 mg RBV. Next day, He had flu-like symptoms. On 2 weeks after treatment, He felt epigastric discomfort, nausea, and vomiting. We rechecked the laboratory examination, level of amylase and lipase was 129 U/L and 113 U/L. We decided to keep on the therapy because of his symptom was not severe, and amylase, lipase level was not too high. But 4 days later, He had severe epigastric pain and nausea. Laboratory test showed amylase and lipase increased up to 217 U/IL and 458 U/L. We checked abdominal ultrasonography but which showed chronic inflammation of liver, no abnormality of pancreas and pancreatic duct. He had no history of pancreatitis, no other medical history and stopped to drink two years ago. We thought PEG-IFN α-2b and RBV was associated with pancreatitis. FEG-IFN α-2b and RBV therapy was stopped and conservative treatment was done with urinastatin 100,000 IU/day. His symptom was resolved and discharged. Pancreatitis did not recurred (Table 1).
DISCUSSION
In the above cases, acute pancreatitis occurred within two weeks after PEG-IFN α-2b and RBV treatment. Patients’ symptoms and laboratory findings were worsened during treatment, improved after discontinuation of PEG-IFN α-2b and RBV. In case 1, we rechallenge IFN α-2b and RBV, and acute pancreatitis recurred. Alcohol abuse, cholelithiasis, endoscopic retrograde cholangiopancreatography, and hypercalcemia are the common risk factors for acute pancreatitis. Neither of the patients abused alcohol, and neither had gallstones, hypercalcemia, or hyperlipidemia. Two patients didn’t have other clear etiologic factors for acute pancreatitis except PEG-IFN α-2b and RBV. The Naranjo adverse drug reaction probability scale is one of the most valid and widely used techniques in clinical practice. The Naranjo’s algorithm total score of case 1 patient was 8 and case 2 patient was 6, which show probable drug adverse reaction [2]. Considering above situation, we conclude that IFN α-2b and RBV is the most probable cause of acute pancreatitis in the patients.
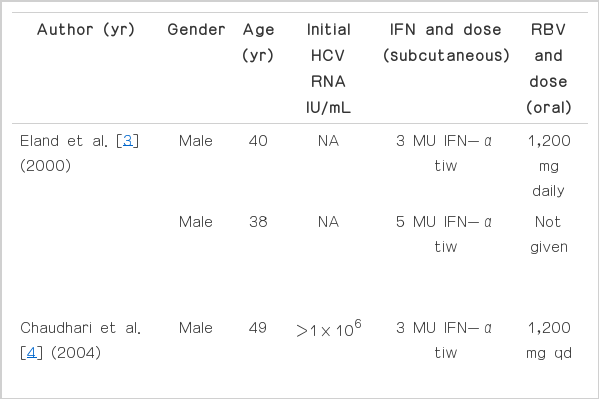
There were some reports about acute pancreatitis associated IFN and RBV treatment. Eland et al. [3] and Chaudhari et al. [4] reported cases that acute pancreatitis developed during IFN α and RBV. Like in our cases, cases of acute pancreatitis associated PEG-IFN α-2b and RBV were report by Cecchi et al. [5], Tahan et al. [6], Ozdogan et al. [7], and Ando et al. [8]. Table 2 shows the features of the other cases that developed acute pancreatitis during CHC therapy. In most cases, rechallenge of IFN and RBV treatment was not performed. But in some cases, including our cases, rechallege was performed and acute pancreatitis was recurred all.
It is difficult to distinguish which drug cause acute pancreatitis between PEG-IFN α-2b and RBV. Because acute pancreatitis occurred during both drugs administration and was improved after stopping both drugs. But some previous studies showed that PEG-IFN α-2b is more probable drug than RBV. Sotomatsu et al. [9] and Tannir et al. [10] reported that acute pancreatitis attributed to use of interferon α in patient of chronic myelogenous leukemia. These studies suggested that PEG-IFN α-2b caused acute pancreatitis more than RBV in our cases.
Mechanism of interferon induced pancreatitis is not known exactly. Treatment with IFN α can result in severe hypertriglyceridemia, a well-described cause of acute pancreatitis. In our cases, however, triglyceride levels were normal range. This mechanism is unlikely to have caused acute pancreatitis in our patients. Ando et al. [8] suggested that PEG-IFN α-2b and RBV may stimulate immune system and cause acute pancreatitis. In our cases, this mechanism may cause acute pancreatitis.
In conclusion, PEG-IFN α-2b and RBV combination therapy is a potential cause of drug-induced pancreatitis in patient. But there is no effective substitute for PEG-IFN α-2b and RBV therapy yet. So this therapy will be continued to be used widely for a while. Therefore, it is important that physicians are alerted to the possibility of IFN α-2b and RBV combination therapy as a cause of pancreatitis. The clinical signs and symptoms of patient should be followed closely. If acute pancreatitis is suspected, physicians should check the blood test about pancreatitis.