INTRODUCTION
Rotational atherectomy (RA) is an interventional technique that uses a high-speed rotational device to ablate heavy calcific plaques and restore vessel patency to enable stent delivery. Compared to standard balloons, RA minimizes vessel wall stretch, reduces barotrauma, and produces smooth lumen. In the drug-eluting stent (DES) era, lesion preparation with the smooth lumen and increased compliance by RA facilitates symmetrical stent expansion resulting in more effective drug delivery. Furthermore, newly developed second-generation DES has similar efficacy compared with first-generation DES and improved safety through greater endothelial cell coverage and less inflammation [1]. Consequently, such factors support the strategy of longer stent deployment which covers the full lesion in comparison to short stent implantation in the past.
According to one previous study which compared the impact of long stent length between first-generation and second-generation DES, major adverse cardiac events (MACEs) increased in the first-generation DES group but not in the second-generation DES group [2].
However, studies analyzing the outcome of patients who received long stent implantation following RA are few in number. In this study, we aimed to compare the clinical outcomes of patients with coronary arterial disease (CAD) who underwent RA with first- and second-generation DES implantation which is ≥32 mm in length.
MATERIALS AND METHODS
1. Subject and procedure
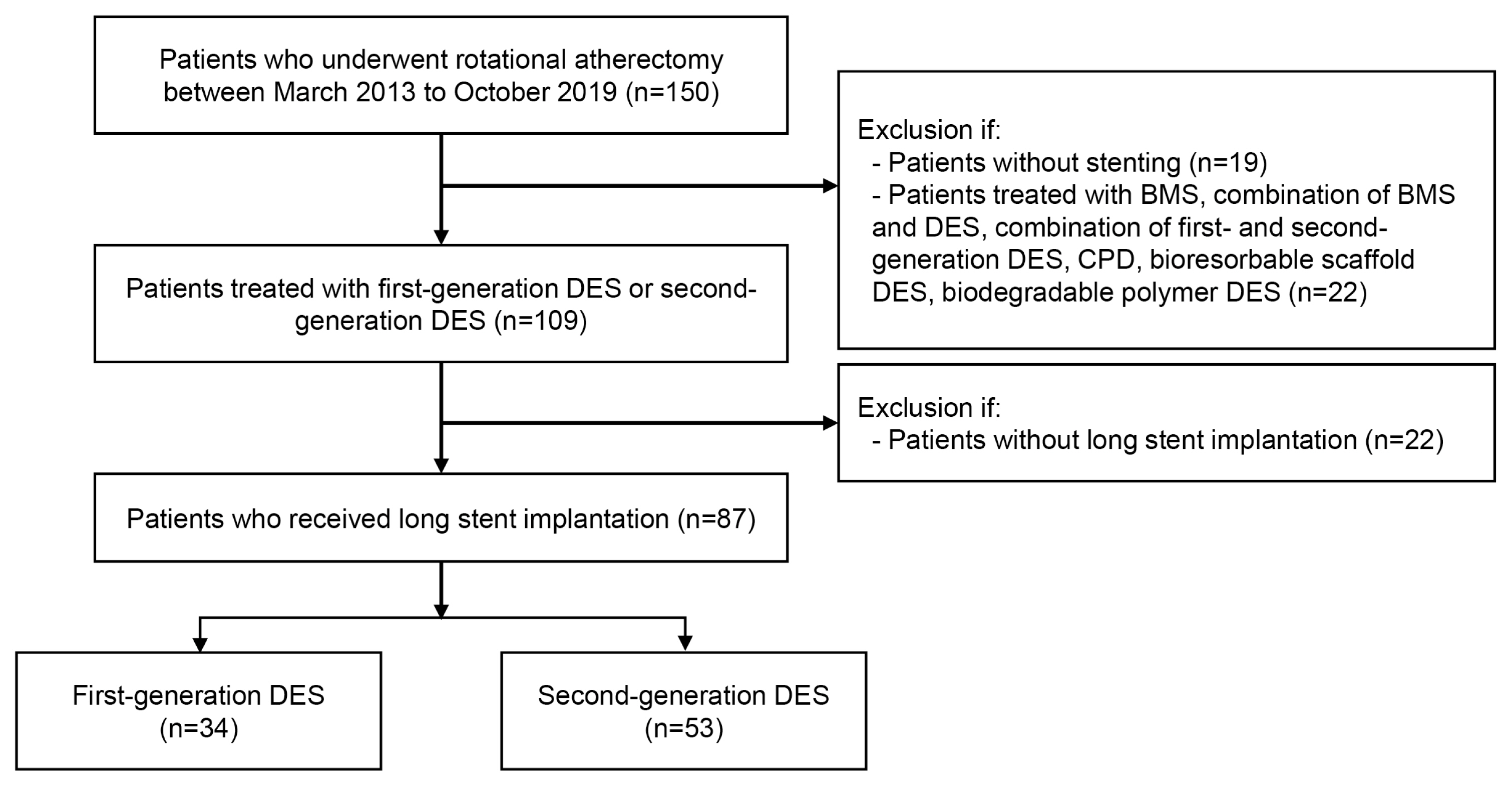
A retrospective cohort study was performed at the single center, Soonchunhyang University Bucheon Hospital, Bucheon, Korea from March 2003 to October 2019. A total of 150 patients with CAD who underwent RA were enrolled in the study. The demographic and procedural characteristics of the patients as well as clinical follow-up records were analyzed. The study was approved by the Institutional Review Board of Soonchunhyang university Bucheon Hospital (IRB file no., 2022-02-006-001) and conformed to the ethical guidelines of the Declaration of Helsinki. The requirement for informed consent from individual patients was omitted because of the retrospective design of this study.
The exclusion criteria included the following: (1) patients without stents or with bare metal stents (BMS), combination of BMS and any type of DES or combination of first- and second-generation DES, cilostazol and paclitaxel dual-coating DES (CPD), bioresorbable scaffold stent, or biodegradable stent. (n=41); (2) patients with DES length less than 32 mm (n=22). As a result, 87 patients eligible for inclusion were enrolled in the study and divided into two groups according to use of first-generation DES or second-generation DES (n=87) (Fig. 1). The first group consisted of patients who received only first-generation DES including sirolimus-eluting Cypher stent (Cordis, Miami Lakes, FL, USA), paclitaxel-eluting Taxus Liberte stent (Boston Scientific, Natick, MA, USA) and Coroflex Please stent (B-Braun, Melsungen AG, Germany). The second group was comprised of patients who received only second-generation DES. The following second-generation DES were used in this study: cobalt-chromium everolimus-eluting Xience Sierra/Alpine/Xpedition stent (Abbott Vascular, Santa Clara, CA, USA), platinum-chromium everolimus-eluting Synergy stent, Promus Element/Premier stent (Boston Scientific; zotarolimus-eluting Endeavor stent, Resolute Integrity/Onyx stent (Medtronic Cardiovascular, Minneapolis, MN, USA); novolimus-eluting DESyne stent (Elixir Medical, Milpitas, CA, USA).
RA (Rotablator system: Boston Scientific Scimed Inc., Maple Grove, MN, USA) was indicated in cases of coronary artery disease with severe calcification and consequent incomplete stent expansion, difficulties in stent or balloon passage. The procedure was conducted according to standard practice. Prior to intervention, patients were treated with an oral loading dose of aspirin and P2Y12 inhibitor (clopidogrel, ticagrelor). Periprocedural anticoagulation with unfractionated heparin (UFH) was administered to patients.
The smallest possible burr was selected at the beginning of the procedure and gradually increased the burr/vessel ratio to 0.5–0.7. Rotational speed was controlled between 140,000 and 180,000 rotations per minute. To prevent slow-flow, a continuous intracoronary infusion of UFH, nitroglycerine, and verapamil was used during RA. After the procedure, all patients received dual antiplatelet therapy with aspirin and one drug among clopidogrel, ticagrelor, or prasugrel. Daily aspirin dosage (100 mg) was recommended to be continued for life and clopidogrel (75 mg once daily), ticagrelor (90 mg twice daily), or prasugrel (10 mg once daily) was prescribed for at least 1 year after the procedure. Regardless of the clinical symptoms of patients, we performed routine follow-up angiography 6–12 months after stent implantation.
2. Definitions and end point
Baseline characteristics included age, sex, body mass index, current smoking, clinical history of hypertension, diabetes mellitus (DM), dyslipidemia, chronic kidney disease (CKD) grade 3 or more, end-stage renal disease on hemodialysis, myocardial infarction (MI), percutaneous cardiac intervention (PCI), and coronary artery bypass graft.
Basically, we identified comorbidities of patients using the International Classification of Diseases-10th revision Clinical Modification codes and the history of medicine prescribed for corresponding diseases.
Procedural characteristics included target vessel, bifurcation, number of stents, stent length, diameter, burr size, procedure duration, usage of intravascular ultrasound (IVUS), chronic total occlusion, slow flow, success, and procedural complications including dissection, perforation, and distal embolization. Stent length was defined as the sum of the length of each implanted stent. Slow flow after RA was defined as thrombolysis in myocardial infarction (TIMI) flow grade ≤2. Angiographic success was defined as grade 3 TIMI flow and final residual stenosis less than 30%.
The primary endpoints of this study were the cumulative 2-year incidence of MACE including all-cause death, cardiovascular death, MI, target vessel revascularization (TVR), stent thrombosis (ST), and acute decompensation of heart failure. Definitions of clinical endpoint were based on the Academic Research Consortium-2 [3]. Cardiovascular death included any death resulting from cardiac causes, all procedure-related death, unwitnessed death, or death of an unknown cause. MI included the cases of periprocedural occurrence, spontaneous occurrence, sudden death, and reinfarction. ST was defined as cases with definite ST or probable ST except possible ST to avoid dilution of events by an overly sensitive definition. TVR was defined as a repeated percutaneous or surgical intervention on the target vessel. Revascularization was performed in cases when the functional study was positive, the patient expressed ischemic symptoms, and when angiographic diameter stenosis was more than 50% or more than 70% without angina.
3. Statistic method
Continuous variables were expressed as means±standard deviation or as median value with interquartile range (IQR). Categorical variables were expressed as percentages. Comparing baseline characteristics, we conducted a chi-square test or Fisher’s exact test for categorical variables and a Student t-test or Mann-Whitney U test for continuous variables. The cumulative incidence of MACE and other adverse events was estimated for 2 years using the Kaplan-Meier curve, log-rank test, and hazard ratio (HR) estimation.
To estimate the adjusted interaction between the type of stent and clinical parameters, we used Cox regression analysis and the result was reported as HR with 95% confidence interval. Univariable Cox analysis was conducted with clinically important variables and variables with P-value <0.1 were entered for multivariable Cox analysis.
In the process of analyzing adjusted interaction with the multivariable Cox regression model, we selected variables considered as conventional risk factors as well as results of univariable Cox analysis. The analytic programs used in this study were IBM SPSS ver. 25.0 (IBM Corp., Armonk, NY, USA), R ver. 3.6.0 (The R Foundation for Statistical Computing, Vienna, Austria; https://www.r-project.org/). Two-sided P<0.05 was considered statistically significant.
RESULTS
1. Baseline characteristics, procedural characteristics
Of the 87 eligible patients, 66 patients (75.9%) completed the 2-year clinical follow-up. Table 1 displays the baseline characteristics. The mean age of the study population was 69±9.1 years with the older mean age in the second-generation DES group (66.7±8.7 versus 70.6±9.0, P=0.051). The proportion of male patients was 49.4% and the rate of current smoking was 16.1%.
The majority of patients had risk factors for coronary artery diseases including dyslipidemia (97.7%), hypertension (83.9%), and DM (71.3%). Although there were no variables with a statistically significant difference, the overall prevalence of comorbidity and the number of patients with a history of coronary artery disease were higher in the second-generation DES group. In addition, the second-generation DES group had more patients with EF <40%. Mean 82.8% of patients underwent PCI with RA for the acute coronary syndrome.
The procedural characteristics are reported in Table 2. The second-generation DES group was associated with a shorter procedure duration (100 [IQR, 70–130] versus 55 [IQR, 30–90], P<0.01) and more common usage of IVUS (32.4% versus 50.9%, P=0.09) without statistical significance. The angiographic success rate was 100% and the complication rate was rare in both groups.
2. Clinical outcomes
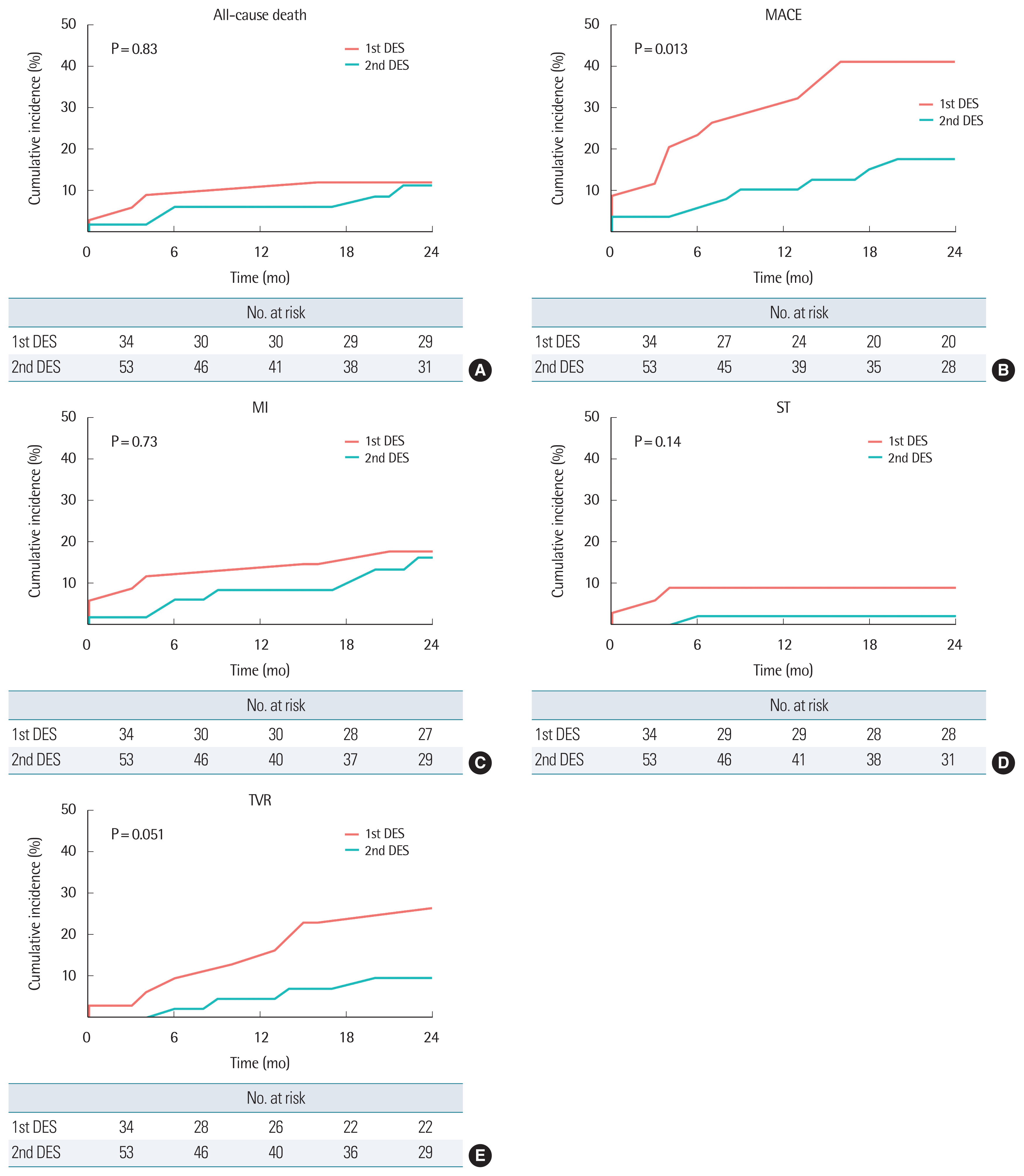
The analyzed clinical outcomes for 2 years are expressed in Fig. 2. All-cause mortality (11.8% versus 9.4%, log-rank P=0.83) and cardiovascular mortality (5.9% versus 5.7%, long-rank P=0.97) was not significantly different in both groups. There was a trend towards a lower rate of MI (17.6% versus 13.2%, log-rank P=0.73), ST (8.8% versus 1.8%, log-rank P=0.14), and TVR (23.5% versus 7.5%, log-rank P=0.051) between the two groups existed. The event of acute decompensation of heart failure (n=1) was rare. However, when combined with MACE, we could identify a significantly reduced incidence rate in the second-generation DES group (41.2% versus 15.1%, P=0.013).
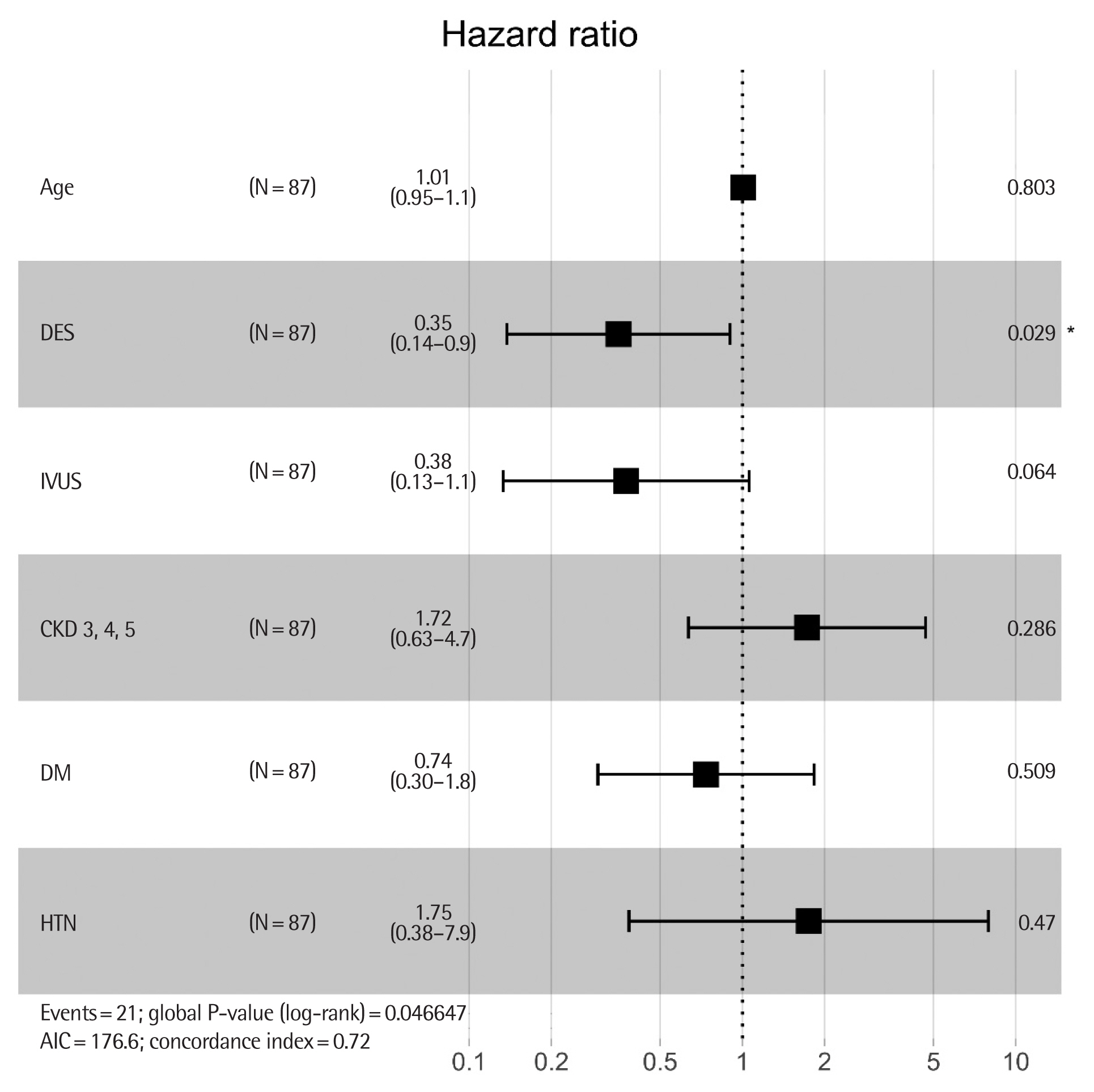
Regarding MACE, we analyzed the effect of the type of DES adjusted with other clinical variables. According to the univariable Cox model, the independently associated factor for MACE was type of DES (HR, 0.35; 95% CI, 0.14–0.83; P=0.02) and usage of IVUS (HR, 0.34; 95% CI, 0.12–0.92; P=0.03). Multivariable analysis including variables considered as conventional risk factors as well as independent factors of the univariable analysis showed that the use of second-generation DES was still independently associated with a lower incidence of MACE. HTN (HR, 1.75; 95% CI, 0.38–7.9; P=0.47) and CKD ≥3 (HR, 1.72; 95% CI, 0.63–4.7; P= 0.29) tended to be associated with MACE (Table 3, Fig. 3).
DISCUSSION
The main finding of the present study is that the second-generation DES group had a statistically significant lower rate of MACE than the first-generation DES group which received ≥32 mm stent followed by RA. With the exception of mortality, the other parameters including MI, TVR, and ST showed a lower tendency in the second-generation DES group. Among the previous studies that analyzed the outcome between the first-generation DES group and the second-generation group treated with RA regardless of stent length, one study reported no clinical difference in 1-year outcome (MACE: 11.9% versus 12.9%, P=1.0) [4]. Another report which compared a 2-year clinical outcome of the same condition identified superior outcome in the second-generation DES group, particularly regarding mortality (MACE: HR, 0.65; 95% CI, 0.42–0.98; P=0.03; all-cause mortality: HR, 0.49; 95% CI, 0.26–0.92; P=0.04) [5]. In SPIRIT (Clinical Evaluation of the Xience V Everolimus Eluting Coronary Stent System in the Treatment of Patients with De Novo Coronary Artery Lesions) trials, the significant superiority of everolimus-eluting stents over paclitaxel-eluting stents regarding mortality was noted as a result of its 3-year study (MACE: HR, 0.71; 95% CI, 0.60–0.85; P=0.003; all-cause mortality: HR, 0.64; 95% CI, 0.49–0.86; P=0.003; MI: HR, 0.64; 95% CI, 0.48–0.85; P=0.002; target lesion failure: HR, 0.71; 95% CI, 0.59–0.85; P=0.0002; ST: HR, 0.45; 95% CI, 0.26–0.78; P=0.0002) [6]. The effect of second-generation DES on clinical outcome improvement seems to be more noticeable with time.
The aforementioned improvements in clinical outcomes are attributed to the features of second-generation DES. First-generation DES was developed to solve the high rate of restenosis in BMS, but long strut exposure time due to antiproliferative drugs resulted in higher incidences of stent thrombosis. Pointing to this problem, second-generation DES was featured with thin strut thickness and biochemically stable polymer [7,8].
In addition, the higher usage of IVUS might have affected the lower incidence of adverse events in the second-generation DES group. Although the routine use of IVUS is still controversial, many studies have revealed that IVUS is associated with improvement in mortality and the incidence of MI, TVR, and ST by providing appropriate reference segment, true vessel size, and plaque morphology [9]. The reference segment identified by IVUS contributed to changes in the stenting strategy to cover full lesions with longer stents [10]. However, the definition of a long stent varies in studies from >13.4 mm to >34 mm [5,6,11–13]. In the current study, we decided to use the most common and recently used definition of ≥32 mm.
The limitations of the current study are as follows: First, this analysis is based on the record of a single center that was collected through a retrospective and non-randomized method. Second, the study cohort was small and there were differences in the number of enrolled patients between the two groups (first-generation group=34 versus second-generation group=53). As the first-generation DES group was smaller in number, the ratio of adverse events was counted higher even with the same number of events. Furthermore, the number of patients who had not completed the 2-year clinical follow-up was not small (n=17, 19.5%), and this could have affected the results of the study. Third, the distribution of the intervention time period of both groups is different. Thus, the higher usage of IVUS and the experience of the operator might have affected the results and the shorter procedural time in the second-generation DES group. Finally, the difference observed in MACE could be the result of an unmeasured component in the present study.
In conclusion, in comparison to the first-generation DES group, the second-generation DES group was associated with a lower rate of MACE for 2 years in patients who underwent RA with long stent implantation.
Although statistically not significant, the incidence of TVR, MI, and ST showed a lower tendency in the second-generation DES group in the same population. Both groups showed similar all-cause mortality and cardiovascular mortality during the 2-year study period.