INTRODUCTION
The ‘burned-out’ phenomenon in germ cell tumors is defined as the presence of an extragonadal germ cell tumor with no evidence of a testicular neoplasm. This condition is different and less common than primary extragonadal germ cell tumors, which represent 2%–5% of adult germ cell malignancies. The origin of extragonadal germ cell tumors remains a matter of debate, and whether such tumors represent metastases of a primary testicular tumor or develop primarily at extragonadal sites is uncertain. Subsequently, Azzopardi et al. [1] firmly established evidence of spontaneous testicular germ cell tumor regression after examining autopsy material from patients who died of widely metastatic testicular germ cell tumors but lacked testicular masses.
We describe spontaneous regression of a primary testicular tumor with metastatic liver and retroperitoneal germ cell tumors, known as the ‘burned-out’ phenomenon.
CASE REPORT
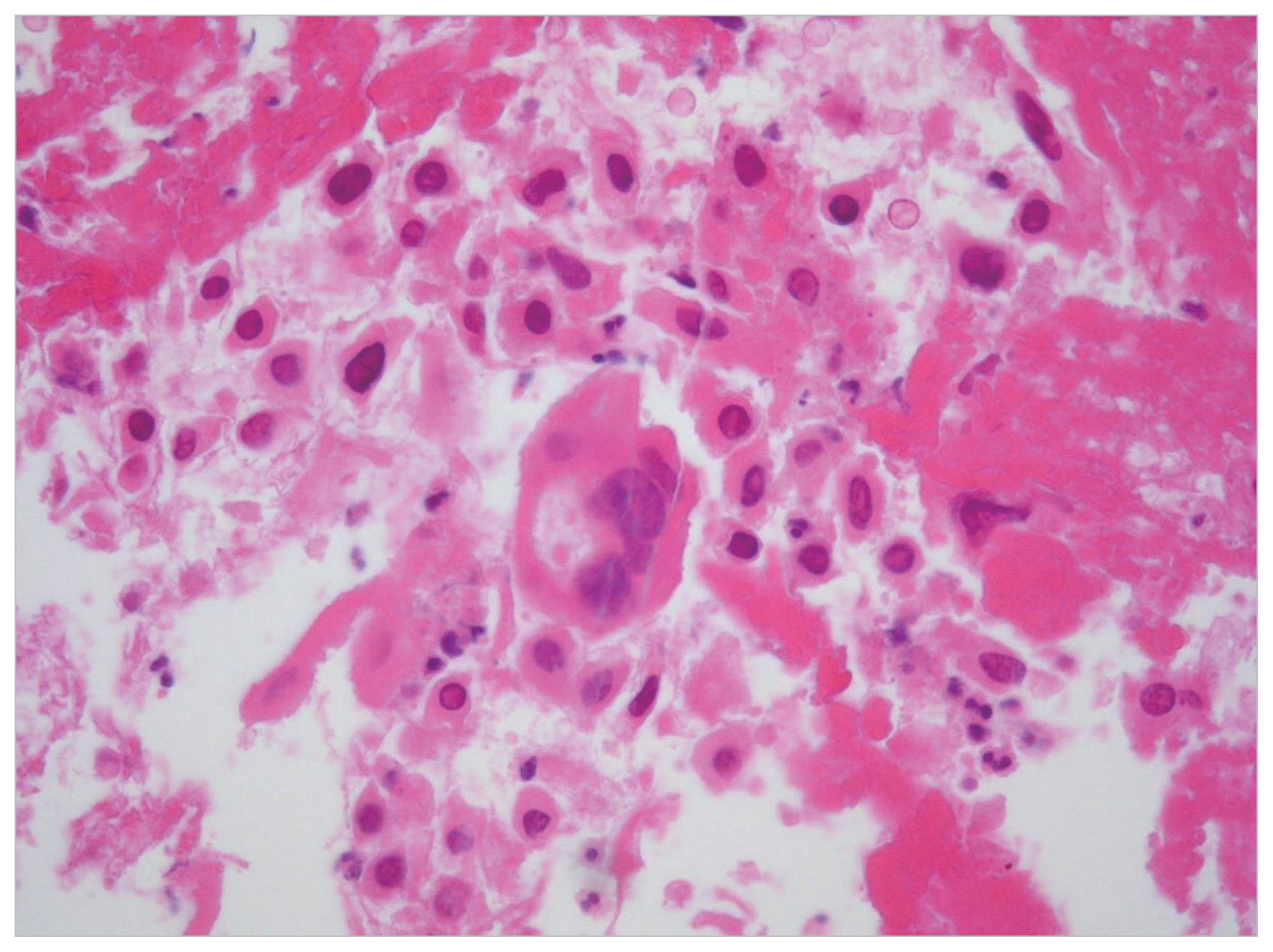
A 33-year-old man presented with left upper quadrant pain and dyspepsia. The patient had a orchiopexy history due to bilateral cryptorchidism. An abdominal computed tomography (CT) scan showed a retroperitoneal mass of 10.7×9.5×16 cm abutting the aorta (Fig. 1). A second CT scan showed a rim-enhancing low-density lesion of the liver and dilatation of the left renal pelvocalyceal system due to the mass effect (Fig. 1). We then examined testicular tumor markers. The patient’s serum β-human chorionic gonadotropin (β-hCG) was beyond the normal range (340.5 mIU/mL), but his α-fetoprotein (α-FP) level was normal. There was no abnormality in the physical examination of the scrotum. However, scrotal ultrasonography showed a heterogeneous echoic intratesticular mass in the left testis. We performed a radical left orchiectomy, and the histological examination showed a well-demarcated nodular scar with large aggregates of Leydig cells in a surrounding atrophied testis (Fig. 2). A sheet of large atypical cells with abundant eosinophilic cytoplasm and moderately pleomorphic nuclei was noted in the periphery of the nodular scar. A needle biopsy of the liver was conducted to exclude seminoma and other germ cell tumors. The histological analysis showed mononuclear and multinuclear tumor cells with abundant eosinophilic cytoplasm in a hemorrhagic and necrotic background (Fig. 3). The tumor cells were positive for pancytokeratin and hCG but negative for cytokeratin-19, vimentin, α-FP, and placental-like alkaline phosphatase. These findings were consistent with metastatic choriocarcinoma. Thus, a diagnosis of spontaneous regression of a germ cell tumor was made. Later, we performed two cycles of BEP (bleomycin+etoposide+cisplatin) chemotherapy, but improvement was not observed on follow-up CT, so we changed the treatment to EMA-CO (etoposide+methotrexate+actinomycin+cyclophosphamide+oncovin). The sizes of the retroperitoneal and hepatic mass lesions decreased, and tumor markers normalized (β-hCG, 0.3 mIU/mL) after seven EMA-CO chemotherapy cycles. We then completely excised the retroperitoneal and hepatic mass lesions, and complete regression was found in both specimens. The patient has been followed for 9 months in the out-patient clinic under a diagnosis of complete remission.
DISCUSSION
A few reports from the early 20th century suggested that testicular germ cell tumors may undergo spontaneous regression. Friedman [2] reported that 23 of 29 patients with primary retroperitoneal germ cell tumors had either regressive changes or an overt tumor of the testis. Ha et al. [3] reported metastatic retroperitoneal seminoma with the ‘burned-out’ phenomenon in testis, but no case has been reported in Korea, with the exception of the present case.
Several immunological and ischemic mechanisms have been suggested to explain this spontaneous regression, but immunological mechanisms appear to be the most likely [4]. This immunological mechanism hypothesis for the regression of a primary testicular tumor suggests that common tumor antigens can be recognized after repeated exposure by cytotoxic T lymphocytes, which are subsequently replaced by fibrosis. However, no consensus exists.
Patients with extragonadal germ cell tumors and spontaneous regression in the testis usually show various clinical symptoms. In this case, our patient complained of left upper quadrant pain and dyspepsia. Although we suspected a testis-originating extragonadal germ cell tumor because of the elevated β-hCG, it was insufficient for the diagnosis. Scrotal ultrasonography may be helpful in such a situation. Comiter et al. [5] reported detection of intratesticular lesions by ultrasonographic examination in patients with retroperitoneal extragonadal germ cell tumors. In particular, microlithiasis is a recognized risk factor and reflects a background upon which testicular germ cell tumors commonly develop [6], but not in this case.
Radical orchiectomy with spontaneously regressing testis is controversial. Although current chemotherapy is largely effective against all type of metastatic testicular germ cell tumors, it is often not effective against primary testicular tumors because of the ‘blood–testis barrier’ [7]. Culine et al. [8] reported that identifying a primary testicular tumor in patients with an extragonadal germ cell tumor is important because it carries the danger of persistent testicular malignancy in up to 50% of patients, despite systemic chemotherapy. Therefore, a radical orchiectomy for an intratubular germ cell tumor in patients with the ‘burned-out’ phenomenon should be considered.
Cisplatinum-based chemotherapy after radical orchiectomy of a primary testicular tumor is the basis of the treatment regimen [9]. The prognosis of patients with an seminomatous extragonadal germ cell tumor in the retroperitoneum is better than that of patients with a nonseminomatous extragonadal germ cell tumor. Schmoll [10] reported that the majority of extragonadal germ cell tumors were of a nonseminomatous pathological type, which have a poor prognosis. If a residual retroperitoneal mass classified higher than stage IIc is present after chemotherapy in cases of nonseminomatous germ cell tumor, it is mandatory to excise the retroperitoneal mass and perform a tumorectomy. Although the patient in our case had a non-seminomatous extragonadal germ cell tumor, complete remission was achieved by completely excising the retroperitoneal and hepatic masses after chemotherapy.
The ‘burned-out’ phenomenon, known as spontaneous regression of a primary testicular tumor, is difficult to recognize and has been incompletely characterized by physicians. Extragonadal germ cell tumors in the retroperitoneum, liver, and other organs should be considered for the ‘burned-out’ phenomenon. Additional studies are needed to identify the exact mechanism and further delineate the nature of the ‘burned-out’ phenomenon.