INTRODUCTION
Disseminated intravascular coagulation (DIC) is a pathological process characterized by the overstimulation of the clotting cascade leading to microthrombi in the small blood vessels [1]. DIC occurs as a complication of another underlying condition including severe infection, malignancy, trauma, and autoimmune disorder such as systemic lupus erythematosus (SLE) [1,2]. Additionally, antiphospholipid syndrome (APS) can be characterized as DIC, in the case of catastrophic APS. Catastrophic APS is an accelerated form of APS that displays features of DIC, resulting in multiorgan failure. Less than 1% of APS develops catastrophic APS, but the condition can be life threatening [3]. As with DIC, most cases of catastrophic APS are preceded by triggering factors including infection, surgery, trauma, and malignancies [2]. However, in some cases, the triggering factor is ambiguous [2]. We report that DIC occurs due to an autoimmune disorder, which may be seronegative catastrophic APS; the patient was treated with weekly rituximab and overcame the disease.
CASE REPORT
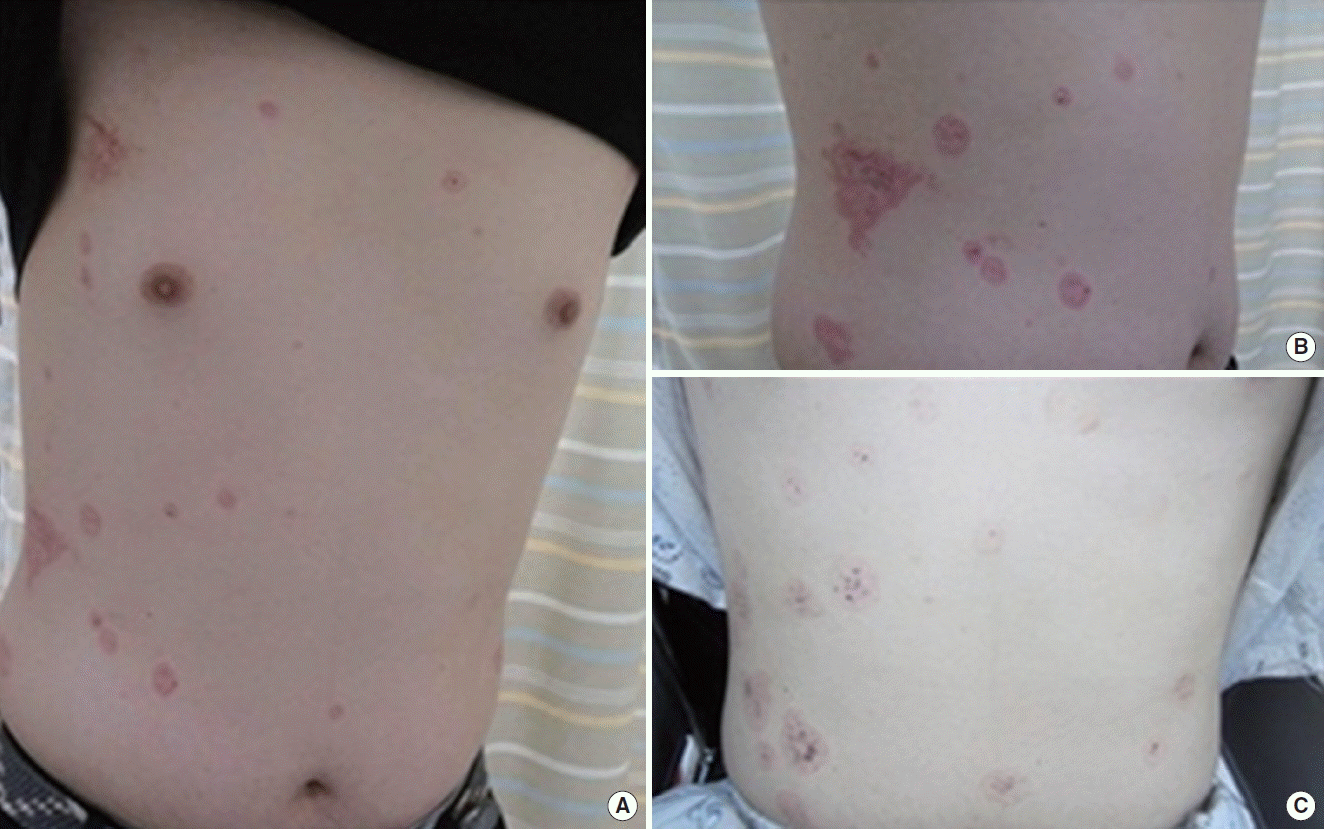
A 20-year-old man was admitted to Seoul National University Hospital for abdominal pain and watery diarrhea on May 1, 2015. He did not have an underlying disease. Seven days prior, he visited the local hospital because of a 3-day history of dry cough. Five days prior, he visited an emergency center due to watery diarrhea. At the hospital, his physical examination and laboratory findings were normal. He was given a diagnosis of ‘acute gastroenteritis’ and went home after supportive care. Four days prior, he complained of sudden tunnel vision, but his symptom was improved soon thereafter. Three days prior, he again visited the hospital because of a persistent cough and took medications including amoxicillin and an antitussive for 3 days. At that time, he had severe watery diarrhea 30 times a day and abdominal discomfort. Additionally, he complained of multiple, red target-like skin lesions on the upper body (Fig. 1). So he visited the hospital again. His laboratory findings showed thrombocytopenia, an abnormal liver function test. A computed tomography (CT) scan of the abdomen showed portal vein thrombosis, hepatic vein thrombosis, superior mesenteric vein thrombosis, renal vein thrombosis, and focal colitis in the ascending colon (Fig. 2). He was brought to the emergency department at our hospital. He complained of general weakness, nausea, vomiting, and encompassing abdominal pain. On physical examination, he had a temperature of 36.1°C, a heart rate of 100 beats/min, a respiratory rate of 18 breaths/min, and a blood pressure of 116/72 mm Hg. Soon after he had sudden hematochezia and a low blood pressure of 71/58 mm Hg. Thus, he was admitted to the intensive care unit (ICU).
Arterial blood gas analysis in the ICU revealed that a pH 7.02 (normal range, 7.38 to 7.46), a pCO2 of 35 mm Hg (normal range, 32 to 46 mm Hg), a pO2 of 480 mm Hg (normal range, 74 to 108 mm Hg), and a HCO3- of 9 mmol/L (normal range, 21 to 29 mmol/L); we, therefore, administered continuous renal replacement therapy to normalize metabolic acidosis. His laboratory findings showed a hemoglobin of 11.4 g/dL (normal range, 13 to 17 g/dL), a white blood cell count of 41,580/μL (normal range, 4,000 to 10,000/μL), a platelet count of 52,000/μL (normal range, 130,000 to 400,000/μL), a C-reactive protein of 9.43 mg/dL (normal range, 0 to 0.5 mg/dL), a total bilirubin of 0.5 mg/dL, an aspartate aminotransferase of 147 IU/L (normal range, 1 to 40 IU/L), an alanine transaminase of 158 IU/L (normal range, 1 to 40 IU/L), a prothrombin time of 34% (normal range, 80% to 120%), an activated partial thromboplastin time of 59.5 seconds (normal range, 26.7 to 37.6 seconds), a fibrinogen of 63 mg/dL (normal range, 180 to 380 mg/dL), a D-dimer of 98.99 μg/mL (normal range, 0 to 0.4 μg/mL), a blood urea nitrogen of 18 mg/dL (normal range, 10 to 26 mg/dL), serum creatinine of 2.26 mg/dL (normal range, 0.7 to 1.40 mg/dL), a lactate dehydrogenase of 7,149 IU/L (normal range, 100 to 225 IU/L), and a haptoglobin of <7 mg/dL (normal range, 30 to 180 mg/dL).
To evaluate infection, hematologic malignancy, and autoimmune diseases, we performed additional laboratory tests. These revealed a low C3 complement of 39 mg/dL (normal range, 70 to 150 mg/dL), a low C4 complement of 7 mg/dL (normal range, 10 to 35 mg/dL), a normal activity of a disintegrin and metalloprotease with thrombospondin type 1 repeats-member 13 (ADAMTS13) at 73% (normal range, 44% to 121%), a normal protein S activity at 72% (normal range, 73% to 150%), and a normal protein C activity at 116% (normal range, 70% to 140%). Additionally, blood culture, enterohemorrhagic Escherichia coli (EHEC) polymerase chain reaction (PCR), rheumatoid factor, antinuclear antibody, anti-neutrophil cytoplasmic antibody, anti-CCP antibody, lupus anticoagulant, anti-cardiolipin antibody, anti-beta 2-glycoprotein antibody, cryoglobulin, direct and indirect Coom’s test, the paroxysmal nocturnal hemoglobinuria test, and bone marrow examination all showed negative findings. Additionally, peripheral blood smear showed normocytic normochromic red blood cells, poikilocytosis, and schistocytes 0.4/high power field.
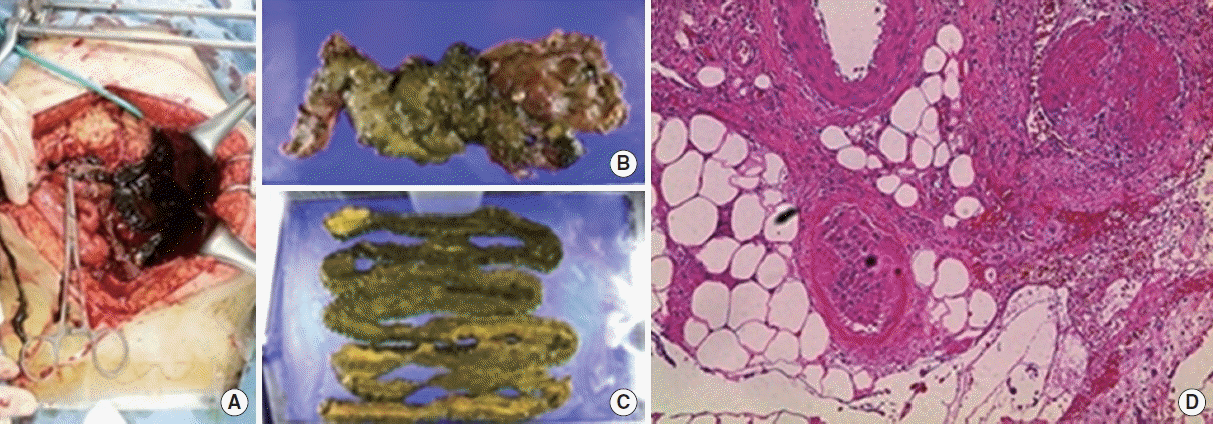
On day 7 of hospitalization, a follow-up abdomen CT was performed to evaluate continuous hematochezia. CT findings revealed the newly developed thrombosis at the left common iliac vein, the left internal vein, the both external iliac vein, and the left femoral vein. Additionally, ischemic colitis of the small bowel with venous thrombosis was found. We performed an inferior vena cava filter insertion for deep vein thrombosis on day 8 of hospitalization and anticoagulation therapy using low-molecular-weight heparin on hospitalization day 13. On day 28 of hospitalization, small bowel segmental resection was performed due to acute bowel perforation caused by ischemic colitis (Fig. 3A). Microscopic examination showed fibrin thrombi in a few vessels and transmural hemorrhagic necrosis (Fig. 3B).
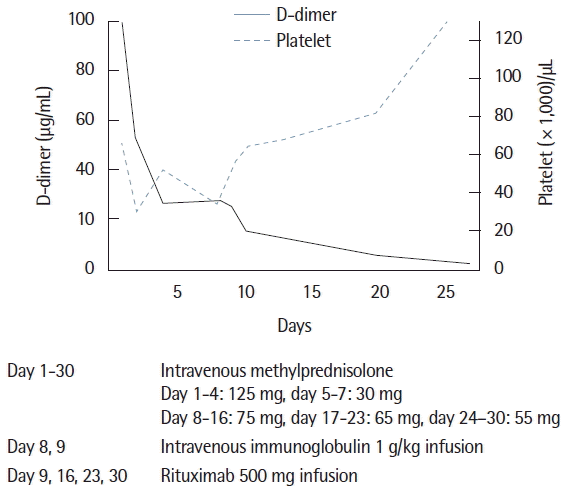
After exclusion of infection and malignancy, the patient received steroid therapy from hospitalization day 1, which was used to treat the autoimmune disorder. However, steroid therapy was not effective in normalizing his abnormal laboratory findings, including the coagulation test and platelet levels. To treat for aggravated thrombocytopenia, intravenous immunoglobulin was administered during hospital day 8 and 9. We suspected steroid refractory autoimmune disorder, therefore, rituximab at 500 mg was infused on hospital days 9, 16, 23, and 30. After rituximab treatment, the platelet count was recovered to over 100,000/μL, and the coagulation test including the D-dimer returned to within normal levels (Fig. 4). The patient was discharged from the hospital on hospitalization day 40.
DISCUSSION
Rituximab, an anti-CD20 monoclonal antibody, is an immune modulator agent that has an effect on lymphoproliferative disorders, autoimmune disorders, and thrombotic microangiopathy (TMA) such as refractory thrombocytopenic thrombotic purpura (TTP) [4,5]. Additionally, in catastrophic APS, it was known that rituximab can be an effective treatment in some cases [6]. However, to our knowledge, this is the first case of seronegative catastrophic APS treated with rituximab in Korea. Catastrophic APS may result from the development of systemic inflammatory response syndrome associated with high levels of cytokine release. Therefore, reducing cytokines from B cells by rituximab may be an effective therapeutic approach [6].
Steroid refractory thrombocytopenia in our patient was recovered by rituximab. The triggering factors in our patient were ambiguous. We excluded lymphoproliferative disorder or other malignancy due to a normal bone marrow exam and CT findings. Additionally, we excluded SLE or other TMA, such as TTP and hemolytic uremic syndrome (HUS) because of negative SLE specific antibodies, normal activity of ADAMTS13, and negative EHEC PCR. Moreover, the coagulation test and D-dimer levels were abnormal. At patient’s initial presentation, we suspected catastrophic APS because the symptoms were characterized by multiple thrombosis developing during a short period of time, similar to catastrophic APS. Additionally, with the exception of seronegative antiphosphopilid antibodies (aPLs), other findings satisfied the criteria for catastrophic APS including three or more organs (skin, liver, small bowel, and kidney) being affected by thrombosis within a short period as well as the evidence of small vessel occlusion found in the pathologic diagnosis at small bowel resection [7].
In a previous report, almost half of the patients who develop catastrophic APS did not have a history of seropositive aPLs. Additionally, in some APS patients, seronegative condition can persist [7–9]. Many possibilities can explain seronegative APS. First, some APS patients may have antibodies to other membrane phospholipids such as phosphatidylserine or phosphatidylethanolamine, which cannot be detected by routine laboratory tests [8,9]. Second, the level of aPLs can fall due to consumption during an acute thrombotic event [7,8]. A previous study reported patients who demonstrated seronegativity for aPLs at the time of thrombotic events, but antibodies were detected later [8].
The differential diagnosis of catastrophic APS includes sepsis, TMA including HUS, TTP, and DIC. All share similar features, however, DIC occurs as a complication of another underlying condition including severe infection, malignancy, trauma, and drug reactions [1]. TMA also shares similar characteristics of thrombocytopenia, schistocytes, renal impairment, and neurological features. However, schistocytosis is a predominant feature in HUS and TTP but is mild or absent in catastrophic APS. Additionally, the coagulation test including D-dimer is usually normal in HUS and TTS [10].
Catastrophic APS is a life-threatening disease, with a mortality rate of approximately 50% at the initial event. Thus, early detection and rapid treatment with anticoagulants, corticosteroids, and intravenous immunoglobulin are important in saving a patient’s life. Additionally, rituximab showed 75% recovery of catastrophic APS and is therefore an effective treatment for refractory catastrophic APS [10].
We report that a 20-year-old man with seronegative catastrophic APS of unknown origin overcame the disorder by being administered weekly rituximab treatment.