INTRODUCTION
Adenosquamous carcinoma (ASC) is a rare histologic subtype of non-small cell lung cancer (NSCLC) [1-4]. According to the World Health Organization (WHO) lung tumor classification criteria, ASC is defined as a carcinoma that shows components of both squamous cell carcinoma (SCC) and adenocarcinoma (ADC), with each component accounting for 10% or more of the whole tumor [5]. Several studies suggested that ASC of the lung is an aggressive tumor that grows rapidly and has worse prognosis than that of ADC or SCC [1-4].
In patients with advanced NSCLC harboring epidermal growth factor receptor (EGFR) mutations, the epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) including gefitinib and erlotinib can be used as first treatment option [6,7]. However, limited numbers of clinical studies to evaluate efficacy of EGFR-TKIs for ASC have been conducted [8-10]. We report a case of an ASC patient with the EGFR mutation, who showed favorable response to EGFR-TKI, and we review about a necessity of EGFR mutation test and efficacy of EGFR-TKIs in ASC patients from the recent studies.
CASE REPORT
A 69-year-old woman with no smoking history visited to our clinic due to long-standing dry cough lasting for a month. She was hospitalized due to hypertensive intracranial hemorrhage 6 years ago, since then she had been treated with hypertension.
On physical examination, blood pressure was 130/80 mm Hg, heart rate was 78 beats/min, respiratory rate was 16 breaths/min, and body temperature was 36.1°C. Lymph node in neck was not palpated and the findings of auscultation were not significant. Complete blood count results were white blood cell 11,220/mm3, hemoglobin 11.4 g/dL, and platelet 579,000/mm3. Chemistry profile, electrolytes and carcinoembryonic antigen was within normal values.
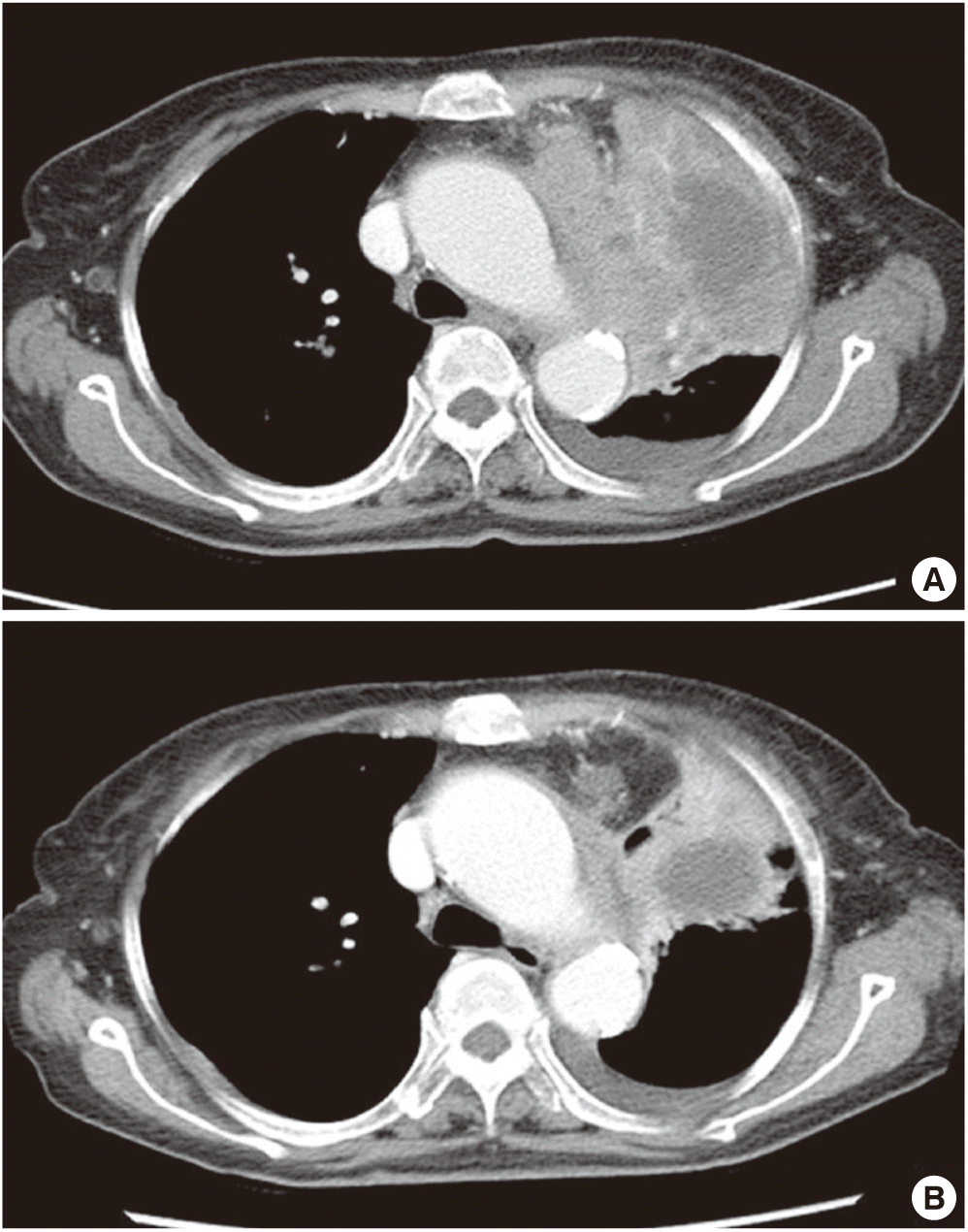
Simple chest radiograph showed a huge mass-like lesion in the left upper lobe (LUL) and multiple small nodules in both lung fields (Fig. 1). These lesions were not seen in the chest radiograph examined six-years ago. Computed tomography (CT) revealed an 8.5×4.7 cm sized heterogeneous enhancing mass in left hilum, multiple hematogenous metastases in both lungs, left pleural effusion, enlarged left supraclavicular lymph node and 2.7 cm sized nodular opacity in left adrenal gland. The left hilar huge mass was abutting left pulmonary artery and ascending aorta, and causing total atelectasis of LUL (Fig. 2).
Flexible bronchoscopy was performed under local anesthesia, which revealed a hyperemic mass obstructing apical segmental bronchus of LUL and other mass obstructing anterior basal segmental bronchus of right lower lobe (RLL). Forcep biopsies were performed 5 times at LUL mass and 2 times at RLL mass.
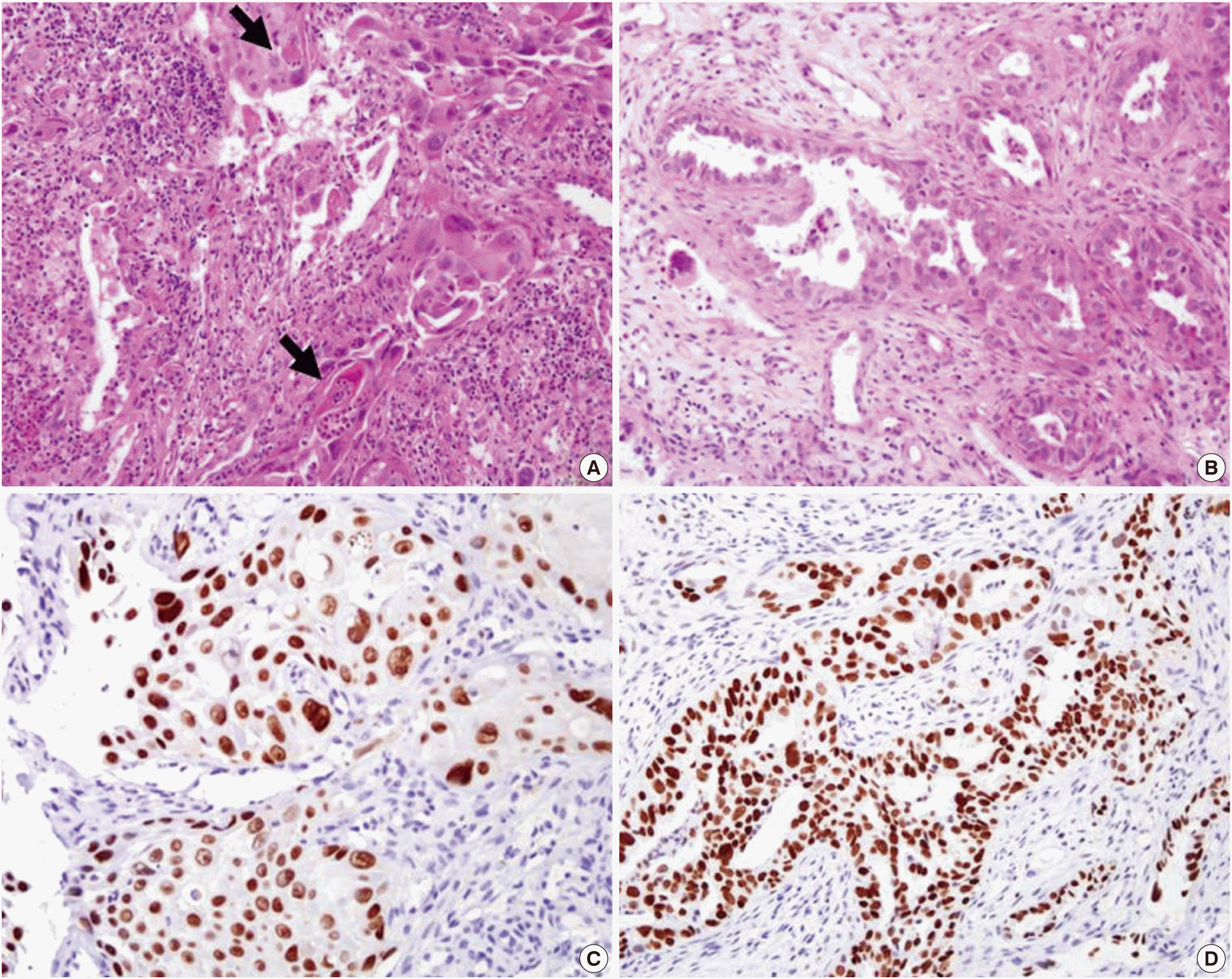
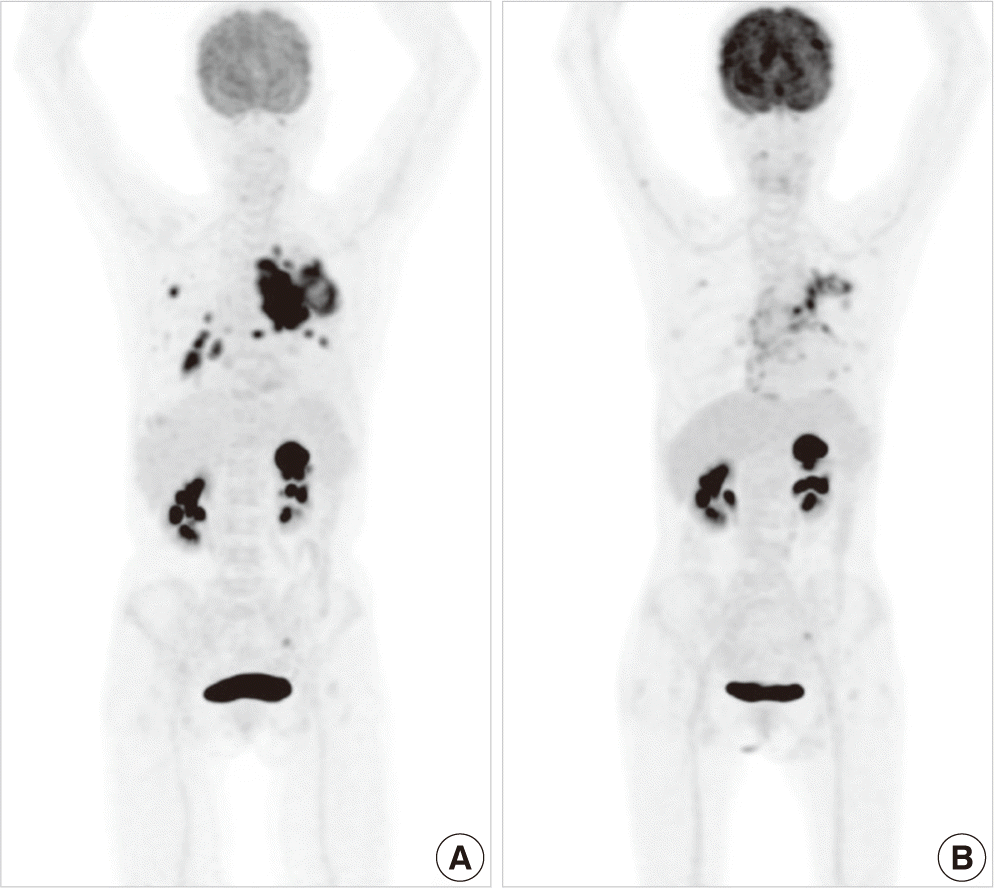
On histologic examination, LUL mass had two distinct histologic appearances. One area showed sheet-like structures composed of atypical cells with abundant eosinophilic cytoplasm and occasional intracellular keratinization, suggesting of SCC. The other area displayed infiltrating glandular structures lined by atypical cells with clear or pale eosinophilic cytoplasm. Some tumor cells had mucin-like materials in their cytoplasm, suggesting of ADC. Immunohistochemical stainings for p63 and TTF-1 were reactive in SCC and ADC areas, respectively (Fig. 3). RLL mass exhibited a pure typical histology of SCC. EGFR mutation testing by direct sequencing method revealed L858R mutation in exon 21. Fluorine 18-labeled fluorodeoxyglucose (FDG)-positron emission tomography (PET)-CT scan revealed abnormal FDG uptake in the left hilar mass, left supraclavicular lymph node, left adrenal gland, and multiple lung nodules (Fig. 4).
We decided to try gefitinib to control advanced ASC with EGFR mutation. She did not show any adverse event related to treatment or cancer itself while taking gefitinib. Subsequent CT scan for response assessment was performed after 2-month of treatment. CT scan revealed decreased size of lung cancer in left hilum (from 8.5×4.7 to 5.3×4.9 cm), decreased number and size of multiple hematogenous metastasis, decreased amount of left pleural effusion, and decreased size of left adrenal gland metastatic nodule (from 2.7 to 1.6 cm), which was corresponded to partial response according to Response Evaluation Criteria in Solid Tumors version 1.1 (Fig. 2).
Another 2 month after CT scan, follow-up PET-CT scan was performed, which revealed decreased size and FDG uptake of primary lesion in left hilum, multiple hematogenous metastases in both lungs, and left adrenal metastasis (Fig. 4). We can’t find any progression during five-month follow-up and she has kept taking gefitinib even now.
DISCUSSION
ASC is a rare histological subtype of carcinoma, comprising less than 5% of all resected NSCLCs [1-4]. According to the WHO lung tumor classification, ASC was histologically categorized as a separate entity which had distinct differences from ADC or SCC [5]. Several studies suggest that ASC of the lung has worse prognosis than that of ADC or SCC [2-4] and clinicopathological study suggest that ASC of the lung is an aggressive tumor that grows rapidly [1]. Subjects of these studies were confined to surgically resected patient, so data concerning advanced or metastasized ASC patients were lacking. Because of limited data about treatment and its heterogenous pathology, how to treat ASC patients is difficult problem, especially advanced or metastasized ASC patients.
Available data about EGFR mutation status in ASC was very limited because of its low incidence. In several studies, 27% to 44% of patients with ASC harbor EGFR-activating mutations in East Asian population [8,11-13], 13% in western populations [14]. The incidence of EGFR mutation in ASC patients was similar to the incidence in ADC patients in the majority of these studies [8,12-14]. Kang et al. [12] reported that EGFR mutations were significantly more frequent in women and never-smokers, on the other hands, Jia and Chen [13] reported that EGFR mutations were not associated with gender, age and smoking history. In an aspect of location of mutation, exon 19 was most frequently affected mutation site and exon 21 was the following [8,11-14]. Similarly, exon 19 deletions and exon 21 L858R point mutations account for approximately 85% of EGFR mutations in NSCLC and are associated with a clinical benefit from EGFR-TKIs [15]. In our case, the patient was women and never-smoker like Kang’s report, and involved mutated location was L858R point mutation in exon 21.
In several studies, efficacy of EGFR-TKIs in the EGFR mutated ASC patients was demonstrated. Shukuya et al. [9] reported a pooled analysis, which included three patients of ASC with EGFR mutation. One of three patients reached partial response (PR), and two had stable disease (SD) with gefitinib treatment [9]. Paik et al. [10] reported 11 ASC patients with EGFR mutation and 8 were evaluable for response. Seven of 8 patients had PR and one had SD. For these 8 patients, median progression free survival (PFS) was 12 months and median overall survival was 29 months [10]. In a recent Chinese study which included 49 ASC patients, 21 ASC patients were conducted EGFR mutation analysis (7 with EGFR mutation and 14 with EGFR wild-type). These 7 EGFR mutated ASC patients underwent EGFR-TKI therapy, then five patients had PR, one had SD, and one had progressive disease. The PFS was 2.4 months and 9.4 months in wild-type and mutation group, respectively. The response rate and the PFS of EGFR mutated ASC patients were similar to that of EGFR mutated ADC patients in this study [8].
Although these studies were retrospectively conducted and included relatively small number of ASC patients, efficacy of EGFRTKIs in EGFR mutated ASC patients is thought to be promising. As of yet, there is insufficient evidence about what is more optimal first-line treatment for EGFR mutated ASC patients within platinium-based doublet chemotherapy or EGFR-TKIs. In our case, we used gefitinib as EGFR-TKI to treat EGFR mutated ASC patient, and she underwent favorable response during 4-month follow-up.
In most clinics, EGFR mutation analysis in ADC are routinely conducted, but not in SCC patients because of low prevalence of EGFR mutations and diminished sensitivity to EGFR TKIs in SCC [9]. Although ASC belongs to a separate histologic entity from ADC and SCC, ASC is consider to have similar character with ADC rather than SCC especially in aspects of incidence of EGFR mutations and responses to EGFR-TKIs, and the ratio of histopathological component (ADC or SCC component) may not have influence the nature of ASC. In summary, the examination of EGFR mutation status can be recommended as important first-step test, and EGFR-TKIs can be considered as first-line treatment option in advanced ASC patients harboring EGFR mutation like ADC patients.