SMS 2011 December;17(2):118-121.
Published online 2011 December 30 |
| Copyright ⓒ 2010 Soonchunhyang Medical Science
|
| Cutaneous Neonatal Lupus Erythematosus Associated with Brain Infarction |
| Soo Young Lee1, Sanghoon Lee2, Jang Yong Jin1, Sung Shin Kim1, Jae Ock Park1, Chang Hwi Kim1
|
| Departments of 1Pediatrics and 2Dermatology, Soonchunhyang University Bucheon Hospital, Soonchunhyang University College of Medicine, Bucheon, Korea |
| Corresponding Author: Sung Shin Kim , Tel: +82-32-621-5401 , Fax: +82-32-621-5016 , Email: kimss@schmc.ac.kr
|

|
ABSTRACT
|

|
|
| Neonatal lupus erythematosus (NLE) is an acquired autoimmune disorder caused by the transplacental passage of maternal autoantibodies, usually anti-Ro/SSA or anti-La/SSB antibodies, and less commonly U1-ribonucleoprotein. NLE usually involves a single organ, but multiple organ involvement has also been reported. Manifestations of NLE may include cutaneous lesions, congenital heart block, hematological diseases (anemia, thrombocytopenia, neutropenia), hepatic diseases (hepatitis, hepatosplenomegaly, cholestasis), and neurological diseases. Neurological involvement is very rare in infants with NLE. Here, we report a 2-day-old female neonate, born to a clinically asymptomatic mother, presenting with cutaneous lupus lesions and brain infarction as a neurological disease. |
|
Keywords: Neonatal lupus erythematosus; Brain infarction |
|

|
INTRODUCTION
|

|
|
| Neonatal lupus erythematosus (NLE) is a rare condition associated with transplacental passage of maternal immunoglobulin G autoantibodies, usually anti-Ro/SSA or anti-La/SSB antibodies, and, less commonly, U1-ribonucleoprotein
[1]
. NLE is sometimes associated with mainly two clinical manifestations, i.e., permanent congenital heart block (CHB) and transient cutaneous lesions. Cutaneous lesions are seen in 15 to 25% of NLE infants
[2]
. The most important organ involvement in NLE infants is the heart, which results in significant morbidity and mortality. Complete heart block is irreversible and requires a cardiac pacemaker
[3]
. CHB occurs in 30% of NLE infants, but both skin lesions and heart block are seen in only 10% of cases
[4]
. Noncardiac extracutaneous manifestations, such as hematological abnormalities, hepatic diseases, and neurological diseases, are uncommon in NLE. Antibody levels tend to disappear by 6 months of age, generally along with clearance of the rash. Most manifestations resolve spontaneously, although CHB is permanent. There have been some previous reports of neurological involvement in NLE. Here, we report a case of cutaneous NLE in an infant with brain infarction without CHB. |

|
CASE REPORT
|

|
|
A 3.52 kg female infant was born after a 39-week uncomplicated pregnancy to a 31-year-old woman with no history of autoimmune disease. The mother also had a healthy 25-month-old boy who was born at 40 weeks of gestation, with a birth weight of 3.2 kg and uneventful prenatal history. A 2-day-old female neonate was transferred to the pediatric clinic for examination of cutaneous lesions seen since her birth. On admission, she was alert, with a body temperature 36.7°C, pulse rate 146 beats per minute, respiratory rate 30 breaths per minute, and blood pressure 60/40 mmHg. At birth, weight, height and head circumference was 3,190 g, 49 cm, 34 cm, respectively. Multiple annular skin lesions were seen in the periorbital area, scalp, trunk, and extremities. These lesions had an erythematosus border with a depressed, ecchymotic central area
(Fig.1)
.
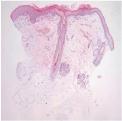
A biopsy from the rash located on the head showed hyperkeratosis, mild upper dermal interstitial infiltration of lymphohistiocytes, and upper dermal blood vessel dilatation
(Fig.2)
. Skin biopsy was suggestive of subacute cutaneous lupus erythematosus.
The results of thoracic, pulmonary, gastrointestinal, and neurological examinations were normal. Laboratory tests revealed a he-moglobin level of 15.5 g/dL, white blood cell count of 17,820/mm3, and platelet count of 378,000/mm3. All results of biochemical tests, including glucose, sodium, potassium, calcium, phosphorus, and magnesium, were within normal limits. Liver function tests, including aspartate aminotransferase, alanine aminotransferase, total bilirubin, and renal function tests, including blood urea nitrogen and creatinine, yielded normal results. Newborn screening tests and hearing screening tests were normal. Serology for syphilis was negative. Anti-nuclear antibody was positive (1:40, speckled pattern). Anti-Ro/SSA and anti-La/SSB antibodies were positive, whereas anti-ribonucleoprotein antibody and anti-double-stranded DNA antibody were negative.
The results of chest radiography, electrocardiography, and transthoracic echocardiography were all normal. Abdominal ultrasonography showed normal results. Cranial ultrasound showed diffusely elevated echogenicity of the white matter, so brain magnetic resonance imaging (MRI) was performed. Brain MRI at 2 weeks of age revealed old infarction in both temporal lobes
(Fig.3)
. However, the patient had no seizures or other abnormal neurological manifestations. So we didn’t perform electroencephalography. The 31-year-old mother had no history of autoimmune disease and was asymptomatic. We recommended that the patient’s mother undergo serological tests but she refused due to a fear of being diagnosed as having autoimmune disease. The infant’s cutaneous lesions were treated with mild topical corticosteroid, and the lesions were improved. There was no clinical evidence of neurological disease in this case. |

|
DISCUSSION
|

|
|
NLE is a model of passive autoimmunity in which maternal anti-Ro/SSA and/or anti-La/SSB antibodies cross the placenta and presumably injure the fetus. In one study, anti-Ro/SSA antibody alone was seen in 62 cases (32%), anti-La/SSB antibody alone was seen in seven cases (4%), both anti-Ro/SSA and anti-La/SSB antibodies were detected in 94 cases (49%), and neither anti-Ro/SSA nor anti-La/SSB antibody were seen in eight cases (4%)
[5]
. There have been some reports of histological documented cases of NLE in which these antibodies were not detected
[6]
.
NLE is a rare disease with an incidence of 1 in every 20,000 live births
[7]
, which presents in the children of women with systemic lupus erythematosus (SLE), Sjögren’s syndrome, and other autoimmune diseases. Approximately half of the mothers with NLE are asymptomatic
[8]
, and there are data supporting a 16 to 24% recurrence rate of NLE in subsequent pregnancies
[9]
. Our patient had typical skin lesions and tests for both anti-Ro/SSA and anti-La/SSB antibodies were positive. The mother of our patient was asymptomatic. However, she refused to undergo blood tests due to a fear of being diagnosed as having SLE or other autoimmune diseases. According to the Research Registry for Neonatal Lupus, only half of the asymptomatic mothers of NLE children show progressive disease, while the majority develop only mild symptoms without life-threatening diseases such as lupus nephritis
[8]
. Counseling is required regarding the risk of additional affected pregnancies and maternal health concerns disease progression. Anti-La/SSB antibody is associated with cutaneous NLE and anti-Ro/SSA influences the development of cardiac injury
[7]
. The Ro antigen is found in the skin, heart, liver, bowel, lung, blood vessels, and brain tissues, which are most often affected in NLE. Clinical manifestations for NLE may include cutaneous lesions, congenital heart block, hematological and hepatic abnormalities, and, rarely, neurological manifestations. The most common manifestations are cutaneous lesions and congenital heart block.
The characteristic findings of cutaneous lesions are annular or round plaques on the face, head, trunk, or extremities. The rash is most commonly detected between 4 and 6 weeks of age, but may be present at birth. The mean duration of the skin lesions is 15 to 17 weeks
[10]
. The cutaneous lesions are most frequently present as an erythema around the periorbital area, not in the malar area, looking like an “owl eye” or “eye mask” appearance
[11]
. These lesions may be aggravated by sunlight but may also be seen in non-sun-exposed areas. The cutaneous lesions disappear spontaneously without any sequelae by 6 months of age, which is associated with the decrease in maternal autoantibody levels in the child’s blood. However, there have been a few reports of cases with sequelae such as persistent telangiectasias, hypopigmentation, and skin atrophy. The classic histopathology of the skin lesions is similar to that of subacute cutaneous lupus, showing keratinocyte damage and superficial mononuclear cell infiltration. Cutaneous lesions tend to resolve spontaneously. But for symptom relief, low-dose topical steroids and photoprotection can be used.
The cardiac manifestations of NLE may include congenital heart block, which is related to fetal exposure to very high titers of anti-Ro/SSA antibody
[12]
, and overwhelming cardiomyopathy that can be none of any conduction disease
[13]
. Irreversible and complete heart block is permanent and requires a pacemaker.
Hematological and hepatic diseases are present in approximately 10% of NLE infants
[6]
, and these may include hepatitis, hyperbilirubinemia, liver failure, and asymptomatic elevation of transaminase level. In most cases symptoms are transient, so the prognosis is good. But very rarely, it may progress to fatal liver failure.
Thrombocytopenia is the most common hematological disease, and neutropenia, anemia, and pancytopenia have also been report-ed in NLE. In most cases, the cytopenia is transient and is not related to morbidity.
Neiman et al.
[10]
reported the development of rheumatic disease later in childhood or adolescence in four of 57 cases of NLE (Hashimoto’s thyroiditis, juvenile rheumatoid arthritis, and Raynaud’s phenomenon). Thus, long-term follow-up is essential in NLE.
Neurological involvements, such as spastic paraparesis, myelopathy, asymptomatic neuroimaging abnormalities, central nervous system (CNS) vasculopathy, and hydrocephalus, are now increasingly recognized as potential manifestations of NLE [14,15]. In 2003, Prendiville et al.
[14]
reported the possibility of CNS involvement based on computed tomography (CT) and ultrasonography of the brain in 10 cases of NLE. CT abnormalities were found in nine of ten cases and ultrasonographic abnormalities were observed in six cases. No CNS symptoms or clinical findings were observed in this cohort with the exception of one case of macrocephaly. In 2007, Boros et al.
[16]
reported hydrocephalus as a new manifestation of NLE, which supported the findings in a previous report regarding hydrocephalus in two female siblings with NLE
[17]
.
The occurrence of seizures is the most commonly reported neurological manifestation of NLE
[18]
. One male NLE infant with residual skin atrophy was reported to have developed a severe seizure disorder at 2 years of age
[14]
. The underlying pathology of neuroimaging abnormalities in NLE is unclear. Based on the typical skin and serologic findings, our patient clearly had NLE. We performed cranial ultrasonography and MRI to examine the occurrence of CNS abnormities in NLE. Brain MRI in our patient
(Fig.3)
showed old infarction in both temporal lobes, which were seen as areas of low signal intensity on T1-weighted axial images (A) and high signal intensity on T2-weighted axial images (B). Diffusion-weighted images showed no evidence of restricted diffusion. Anti-phospholipid antibodies were shown be associated with thrombosis and to cause cerebrovascular accidents in cases of pediatric SLE
[19]
. This said, we took a blood sample on admission, but our study had the limitation that we did not assay for the presence of anti-phospholipid antibodies. In our case, there were no risk factors for brain infarction including bacterial meningitis, inherited or acquired coagulopathies, trauma, hypoglycemia and perinatal asphyxia. So we assumed that NLE was responsible for brain infarction in this case. Long-term follow-up is indicated in such cases regardless of whether the patient shows seizures or abnormal neurological symptoms.
Here, we reported a case of NLE with typical cutaneous lesions and brain infarction, which has not been reported previously. As central nervous system involvement in NLE is being reported with increasing frequency, brain imaging studies are worthwhile in infants diagnosed with this condition. Such neonates showing neurological involvement should be followed up closely. |
|

|
FIGURES
|

|
|

|
Fig.1
Erythematosus skin lesions around the eyes and annular plaques on the face and head. |

|
|
Fig.2
The epidermis shows hyperkeratosis and mild lymphoid infiltration that is primarily superficial (H&E, ×40). |

|

|
Fig.3
Brain magnetic resonance imaging showed old infarction in both temporal lobes, seen as regions of low signal intensity on T1-weighted axial images (A) and high signal intensity on T2-weighted axial images (B). Diffusion-weighted imaging showed no evidence of restricted diffusion (C). |

|
|
| |

|
REFERENCE
|

|
|
|
1.
|
Fioretti A, Eibenstein A, Fusetti M. New trends in tinnitus management. Open Neurol J 2011;5:12-7. |
|
2.
|
Silverman E, Jaeggi E. Non-cardiac manifestations of neonatal lupus erythematosus. Scand J Immunol 2010;72:223-5. |
|
3.
|
Buyon JP, Hiebert R, Copel J, Craft J, Friedman D, Katholi M, et al. Autoimmune-associated congenital heart block: demographics, mortality, morbidity and recurrence rates obtained from a national neonatal lupus registry. J Am Coll Cardiol 1998;31:1658-66. |
|
4.
|
Boh EE. Neonatal lupus erythematosus. Clin Dermatol 2004;22:125-8. |
|
5.
|
Kobayashi R, Mii S, Nakano T, Harada H, Eto H. Neonatal lupus erythematosus in Japan: a review of the literature. Autoimmun Rev 2009;8:462-6. |
|
6.
|
Kim KR, Yoon TY. A case of neonatal lupus erythematosus showing transient anemia and hepatitis. Ann Dermatol 2009;21:315-8. |
|
7.
|
Lee LA. Neonatal lupus erythematosus: clinical findings and pathogenesis. J Investig Dermatol Symp Proc 2004;9:52-6. |
|
8.
|
Rivera TL, Izmirly PM, Birnbaum BK, Byrne P, Brauth JB, Katholi M, et al. Disease progression in mothers of children enrolled in the Research Registry for Neonatal Lupus. Ann Rheum Dis 2009;68:828-35. |
|
9.
|
Izmirly PM, Rivera TL, Buyon JP. Neonatal lupus syndromes. Rheum Dis Clin North Am 2007;33:267-85, vi. |
|
10.
|
Neiman AR, Lee LA, Weston WL, Buyon JP. Cutaneous manifestations of neonatal lupus without heart block: characteristics of mothers and children enrolled in a national registry. J Pediatr 2000;137:674-80. |
|
11.
|
Lee LA. The clinical spectrum of neonatal lupus. Arch Dermatol Res 2009; 301:107-10. |
|
12.
|
Jaeggi E, Laskin C, Hamilton R, Kingdom J, Silverman E. The importance of the level of maternal anti-Ro/SSA antibodies as a prognostic marker of the development of cardiac neonatal lupus erythematosus a prospective study of 186 antibody-exposed fetuses and infants. J Am Coll Cardiol 2010;55:2778-84. |
|
13.
|
Nield LE, Silverman ED, Smallhorn JF, Taylor GP, Mullen JB, Benson LN, et al. Endocardial fibroelastosis associated with maternal anti-Ro and anti-La antibodies in the absence of atrioventricular block. J Am Coll Cardiol 2002;40:796-802. |
|
14.
|
Prendiville JS, Cabral DA, Poskitt KJ, Au S, Sargent MA. Central nervous system involvement in neonatal lupus erythematosus. Pediatr Dermatol 2003;20:60-7. |
|
15.
|
Caba?as F, Pellicer A, Valverde E, Morales C, Quero J. Central nervous system vasculopathy in neonatal lupus erythematosus. Pediatr Neurol 1996;15:124-6. |
|
16.
|
Boros CA, Spence D, Blaser S, Silverman ED. Hydrocephalus and macrocephaly: new manifestations of neonatal lupus erythematosus. Arthritis Rheum 2007;57:261-6. |
|
17.
|
Nakayama-Furukawa F, Takigawa M, Iwatsuki K, Sato N, Sato H. Hydrocephalus in two female siblings with neonatal lupus erythematosus. Arch Dermatol 1994;130:1210-2. |
|
18.
|
Lin SC, Shyur SD, Li-Hsin-Huang, Wu JY, Ma YC. Focal seizures as an unusual presentation of neonatal lupus erythematosus. Asian Pac J Allergy Immunol 2005;23:61-4. |
|
19.
|
Harel L, Sandborg C, Lee T, von Scheven E. Neuropsychiatric manifestations in pediatric systemic lupus erythematosus and association with antiphospholipid antibodies. J Rheumatol 2006;33:1873-7. |
|
|
|