INTRODUCTION
Pulmonary spindle cell carcinoma (SpCC) consists of malignant spindle cells in fascicular or storiform patterns without differentiated carcinomatous elements [1]. Its incidence is 0.2% to 0.3% among all primary pulmonary malignancies. Bronchial carcinoid tumors are an uncommon group of pulmonary neoplasms characterized by neuroendocrine (NE) differentiation. NE tumors are frequently associated with synchronous or metachronous secondary primary malignancies, with an incidence rate of up to 55% [2]. Here, we describe SpCC of the lung diagnosed and surgically removed 6 months after treatment of a pulmonary carcinoid tumor.
CASE REPORT
A 61-year-old man was admitted to Chungnam National University Hospital with a left upper lobe mass that was incidentally detected on chest radiography during a routine check-up. He had been a smoker for 17 years (0.5 pack per day) but had quit smoking when he was 43 years old. He did not have a family history of any cancer. Chest computed tomography (CT) revealed a 7 mm endobronchial lesion in the lingular division of the left upper bronchus (Fig. 1A). Bronchoscopic biopsy was performed on the fungating mass enclosing the left lingular segment (Fig. 1B, C), which revealed a carcinoid tumor with a uniform gland-like structure and expression of NE markers such as synaptophysin and chromogranin (Fig. 1D–F). No hypermetabolism was detected on positron emission tomography-CT (PET-CT). Thus, the preoperative clinical stage was stage IA (T1a, N0, M0), and left lingular segmentectomy was performed.
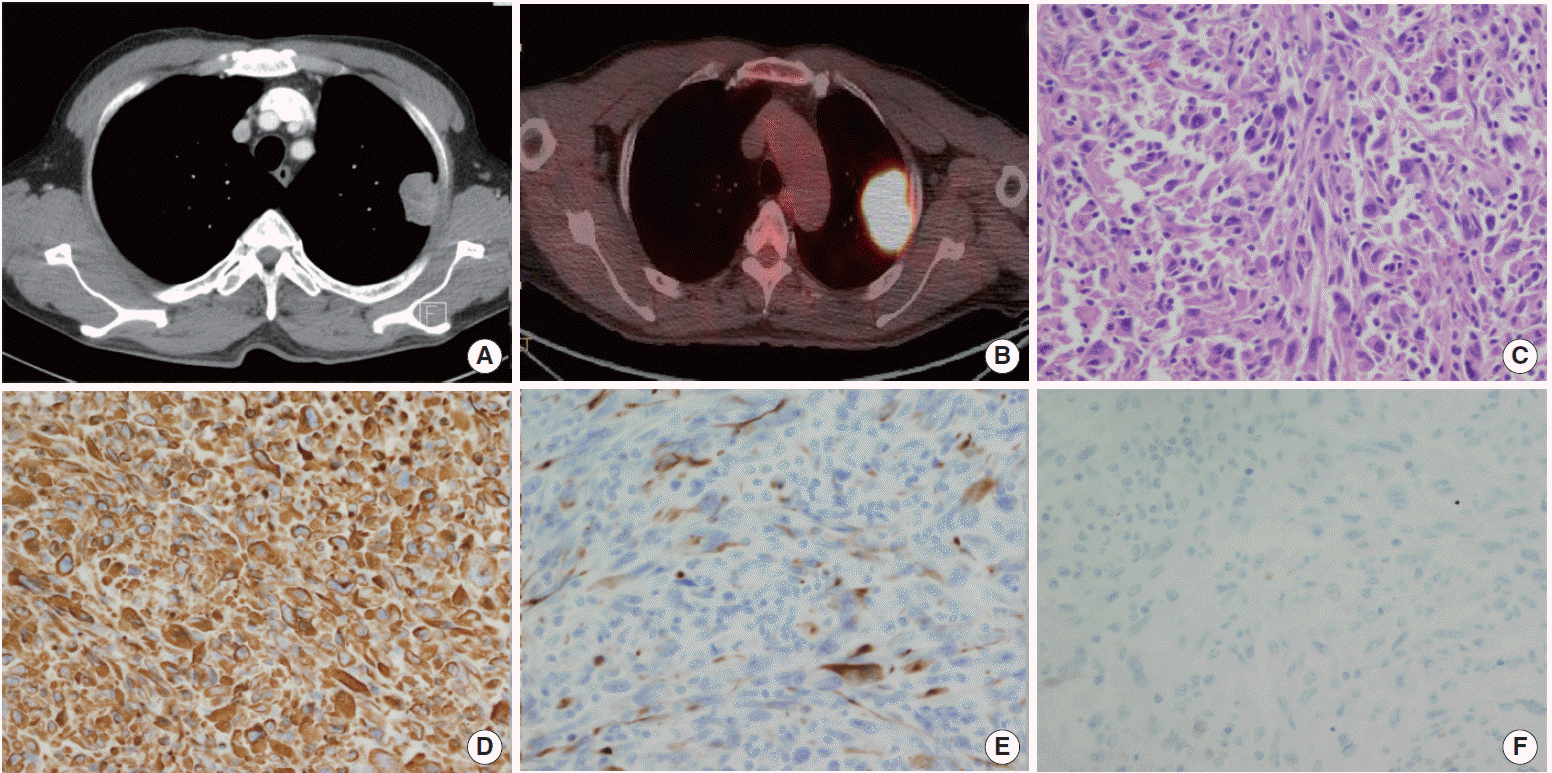
Unexpectedly, on follow-up chest CT performed 6 months postoperatively, we identified a new left upper lobe mass measuring 4.5×4.0 cm (Fig. 2A). On PET-CT examination, a hypermetabolic lesion was found directly invading the chest wall without distant metastasis (Fig. 2B). Percutaneous CT-guided needle biopsy was performed, and microscopic examination revealed spindle-shaped tumor cells with variable amounts of cytoplasm. The pathologic findings were consistent with SpCC or synovial sarcoma (Fig. 2C). The clinical stage was IIB (T3, N0, M0), and left upper lobectomy was performed.
The pathologic stage was determined to be IIB (pT3, N0, M0), and the cancer showed parietal pleural involvement with no lymph node invasion. Immunohistochemistry (IHC) examination showed strong positive results for vimentin and cytokeratins (CKs) such as CK7, CK8/18, and CK19 but negative results for calretinin (Fig. 2D–F). It was difficult to determine whether the tumor was SpCC or synovial sarcoma based solely on the results of the pathologic examination. Radiological findings indicating that the tumor had originated from lung parenchyma favored a diagnosis of SpCC, because pulmonary synovial sarcomas almost arise from the pleura [3]. We recommended adjuvant chemotherapy, but the patient and his family were reluctant for him to receive chemotherapy. At present, 12 months after the second operation, he remains free of recurrence.
DISCUSSION
Pulmonary carcinoid tumors are rare tumors that reportedly account for only 0.4% to 3% of all resected lung tumors and approximately 25% of all carcinoid tumors [1]. NE tumors are often associated with secondary primary malignancies. This may be due to certain mutations associated with multiple endocrine neoplasms or the effects of neuropeptides secreted from NE tumors, among other possibilities [2]. In this case, the primary carcinoid tumor led to extremely rare SpCC of the lung.
Sarcomatoid carcinomas of the lung are also rare tumors, accounting for 0.3% to 1.3% of all malignant lung tumors, and SpCC, which consists of only spindle cell-shaped tumor cells, is even rarer [4]. SpCC is more common in males than females, in smokers, and in patients between 50 and 80 years of age [5]. Definite diagnoses may only be made via IHC analyses of resected tumors. Due to its rarity, the nature of SpCC is not clear because few large studies have been performed [5]. However, these tumors are generally characterized by a more aggressive outcome than those of other types of non-small-cell lung cancer, even during the early stages [6]. Occurrence of a second tumor shortly (<2 years) after the first primary tumor, older age, and incomplete tumor resection are associated with a poor prognosis [7]. In our case, occurrence of SpCC just 6 months after surgical removal of a carcinoid tumor indicated a very aggressive and poor prognosis.
There is one previous case report of a high-grade SpCC after treatment of a primary pulmonary collision carcinoid tumor and another report of SpCC of the lung after treatment of a large cell NE carcinoma [1,8]. To the best of our knowledge, this is the first report of metachronous SpCC following treatment of a primary carcinoid tumor.
SpCC closely resembles synovial sarcoma morphologically, as both carcinomas have spindle-shaped tumor cells. Their overlapping IHC profiles make them even more difficult to differentiate. SpCC is usually strongly positive for vimentin and reacts positively to cytokeratins [9]. On the other hand, synovial sarcomas are nearly uniformly positive for cytokeratin, epithelial membrane antigen, b-cell lymphoma-2, and vimentin and negative for S-100, desmin, and smooth muscle actin [10]. In the present case, the tumor was strongly positive for vimentin as well as CK7, CK8/18, and CK19. However, these findings could not be used to definitively differentiate between SpCC and synovial sarcoma. Further clinical and radiological findings suggest that the tumor originated in the lung parenchyma; hence, because pulmonary synovial sarcomas almost always arise from the pleura, we diagnosed this tumor as SpCC [3].
Although metachronous pulmonary carcinoid tumors in association with SpCC may be extremely rare, SpCC growth can be significantly rapid and the general prognosis dismal. Early detection and appropriate treatment are crucial. Hence, studies on the risk factors of, predictive markers for, and relationships among metachronous cancers would be valuable. Furthermore, clinicians should be aware of the possibility of synchronous or metachronous SpCC in association with carcinoid tumors.